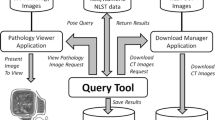
Abstract
From 2002–2004, the Lung Screening Study (LSS) of the National Lung Screening Trial (NLST) enrolled 34,614 participants, aged 55–74 years, at increased risk for lung cancer due to heavy cigarette smoking. Participants, randomized to standard chest X-ray (CXR) or computed tomography (CT) arms at ten screening centers, received up to three imaging screens for lung cancer at annual intervals. Participant medical histories and radiologist-interpreted screening results were transmitted to the LSS coordinating center, while all images were retained at local screening centers. From 2005–2007, all CT exams were uniformly de-identified and delivered to a central repository, the CT Image Library (CTIL), on external hard drives (94%) or CD/DVD (5.9%), or over a secure Internet connection (0.1%). Of 48,723 CT screens performed, only 176 (0.3%) were unavailable (lost, corrupted, compressed) while 48,547 (99.7%) were delivered to the CTIL. Described here is the experience organizing, implementing, and adapting the clinical-trial workflow surrounding the image retrieval, de-identification, delivery, and archiving of available LSS–NLST CT exams for the CTIL, together with the quality assurance procedures associated with those collection tasks. This collection of CT exams, obtained in a specific, well-defined participant population under a common protocol at evenly spaced intervals, and its attending demographic and clinical information, are now available to lung-disease investigators and developers of computer-aided-diagnosis algorithms. The approach to large scale, multi-center trial CT image collection detailed here may serve as a useful model, while the experience reported should be valuable in the planning and execution of future equivalent endeavors.




Similar content being viewed by others
References
Gohagan J, Marcus P, Fagerstrom R, Pinsky P, Kramer B, Prorok P, for The Lung Screening Study Research Group: Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph (The Lung Screening Study of the National Cancer Institute). Chest 126:114–121, 2004
Hillman BJ: Economic, legal, and ethical rationales for the ACRIN National Lung Screening Trial of CT screening for lung cancer. Acad Radiol 10:349–350, 2003
Gohagan JK, Prorok PC, Hayes RB, Kramer BS, and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team: The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials 21(6 Suppl):251S–272S, 2000
Moore SM, Gierada DS, Clark KW, Blaine GJ: Image quality assurance in the Prostate, Lung, Colon, and Ovarian (PLCO) Cancer Screening Trial Network of the National Lung Screening Trial. J Digit Imaging 18(3):242–250, 2005
Clark KW, Gierada DS, Moore SM, et al: Creation of a CT image library for the Lung Screening Study of the National Lung Screening Trial. J Digit Imaging 20(1):23–31, 2007
Cagnon CH, Cody DD, McNitt-Gray MF, Seibert JA, Judy PF, Aberle DR: Description and implementation of a quality control program in an imaging-based clinical trial. Acad Radiol 13(11):1431–1441, 2006
Moore SM, Maffitt DR, Blaine GJ, Bae KT: A workstation acquisition node for multi-center imaging studies. SPIE Medical Imaging 2001, PACS and Integrated Medical Information Systems: Design and Evaluation 4323:271–277, 2001
Washington University in Saint Louis—Polycystic Kidney Disease Treatment Network (PKD-TN). Available at http://www.pkd.wustl.edu/pkd-tn. Accessed 24 June 2008
Washington University in Saint Louis—Silent Infarct Transfusion(SIT) Study. Available at http://sitstudy.wustl.edu. Accessed 24 June 2008
National Cancer Institute—National Cancer Imaging Archive (NCIA). Available at http://ncia.nci.nih.gov. Accessed 24 June 2008
Jannin P, Krupinski E, Warfield S: Validation in medical image processing (guest editorial). IEEE Trans Med Imag 25(11):1405–1409, 2006
National Cancer Institute—National Cancer Imaging Archive (NCIA). Reference Image Database Resource (RIDER) and Plans for a Public-Private Partnership (white paper). Available at http://ncia.nci.nih.gov/ncia/collections (under RIDER). Accessed 24 June 2008
Armato SG, McLennan G, McNitt-Gray MF, et al: Lung Image Database Consortium: developing a resource for the medical imaging research community. Radiology 232:739–748, 2004
National Cancer Institute—Cancer Biomedical Informatics Grid (caBIG). Available at https://cabig.nci.nih.gov. Accessed 24 June 2008.
Saltz J, Oster S, Hastings S, et al: caGrid: design and implementation of the core architecture of the cancer Biomedical Informatics Grid. Bioinformatics 22(15):1910–1916, 2006
Acknowledgements
This research was supported by contracts from the Division of Cancer Prevention, National Cancer Institute (NCI), NIH, DHHS. The authors thank Drs. Christine Berg, LSS-NLST Project Officer, and John Gohagan, former LSS-NLST Project Officer, Division of Cancer Prevention, National Cancer Institute; the Screening Center (Appendix A) investigators, coordinators, and staff of the National Lung Screening Trial (NLST); Mr. Tom Riley and staff, Information Management Services, Inc., and Ms. Brenda Brewer and staff, Westat, Inc, for their support and assistance. The Westat LSS component is supported by NCI Contract NO1-CN-25476. We thank Drs. Richard Fagerstrom and Timothy Church for their reviews of a preliminary version of the manuscript. We also thank our image viewers, without whom the CTIL would have been seriously delayed: Angelica Cosas, Patricia Rueweler, Rochelle Williams, Dr. Sooah Kim, Dr. Miyoung Kim, and Dr. Yuting Liang. The CTIL gratefully acknowledges Merge Healthcare’s generous contribution of the FUSION Server and its continued support under their research agreement with the Mallinckrodt Institute of Radiology. Most importantly, we acknowledge the LSS participants for their contributions to making this study possible.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A
Appendix B: CT Parameters
Researchers using the CTIL will require access to the attributes that are found in the DICOM images. These describe the techniques used for each acquisition as well as the equipment that is used. These attributes, from each image series of each CTIL exam, have been forwarded to Westat in order that Westat might readily respond to a researcher’s formal request for CTIL images by determining which exams will satisfy the researcher’s needs; otherwise, each request involving these attributes would require the extra step of first searching the CTIL database.
Table 4 lists DICOM attributes for values of interest during the screening trial and for secondary analysis. Not all LSS scanners provide values for each of these attributes. In some cases, the attribute stored in the database is actually calculated from other data in the DICOM image. In other cases, the images do not contain sufficient information to calculate or infer those values. The value stored in the CTIL database for each attribute is dependent on the vendor. For example, a manufacturer may provide a value for table rotation but not a value for collimation; or, those values may be entirely absent (Table 4)
Note 1: in order to calculate ‘effective mAs’ (=mAs/pitch), one needs to first determine mAs and pitch. Both of those required extensive reading of DICOM conformance statements and explanations from the manufacturers. Values needed to determine mAs and/or pitch are sometimes stored in private attributes. Discussions with the manufacturers’ engineers allowed us to interpret those private attributes and extract the information necessary to determine effective mAs, mAs and pitch.
Note 2: values for pitch were never found directly in a public DICOM attribute. In the Philips equipment, the value for pitch was found in a private attribute. For GE scanners, a text string in a private attribute stored a coded value used to look up the pitch. The Siemens scanners did not record pitch; we performed an inverse calculation based on the effective mAs stored by the equipment and our determination of mAs from exposure and current.
The Toshiba CT devices store several parameters in private elements that must be combined to compute pitch. The Toshiba CT system creates a private attribute with a binary object that includes a wide range of data. Among other things, these data include the required CT pitch information but also PHI captured by the modality. Passing that attribute from the Toshiba system through the laptop to the CTIL would result in PHI disclosure; omitting that data would exclude the important CT pitch information. This problem was addressed by modifying the laptop software to interpret the Toshiba binary object, extract the required pitch information, and encode the pitch information in a separate private attribute. The original Toshiba private attribute could then be safely deleted. This is the only instance where the laptop de-identification software was modified to interpret data specific to a manufacturer or device model. All other manufacturer specific data (non PHI) were interpreted with custom software maintained at the CTIL.
Note 3: pixel spacing (reconstruction interval) was computed from Image Position (0020 0032) rather than just extracted from (0028 0030). This provided a backup mechanism to allow us to check for uniformity of slice location to make sure that slices were not missing. This is best done by examining Image Position (0020 0032) and not making assumptions about Instance Number (0020 0013).
Note 4: scanner ID was a scanner identifier copied from a cross-reference table of identifiers built from the DICOM attributes Institution (0008,0080), Station Name (0008, 1010), Manufacturer (0008,0070), Manufacturer’s Model Name (0008, 1090), and Software Version (0018, 1020). The table had been built from information provided by LSS medical physicists for known scanners for which the physicists had routinely supervised calibrations as part of LSS quality assurance. If the attributes in the DICOM header of any CT exam delivered to the CTIL could not be matched in this table, the originating site was questioned because the exam seemed to have come from a scanner not being monitored for quality assurance. Such exceptions were fewer than ten; and most of these cases were caused by scanner software upgrades of which the CTIL had not been informed.
Rights and permissions
About this article
Cite this article
Clark, K.W., Gierada, D.S., Marquez, G. et al. Collecting 48,000 CT Exams for the Lung Screening Study of the National Lung Screening Trial. J Digit Imaging 22, 667–680 (2009). https://doi.org/10.1007/s10278-008-9145-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10278-008-9145-9