Abstract
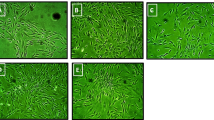
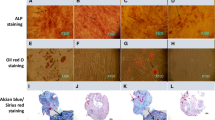
The objectives of this study were to isolate and culture dental pulp stem cells (DPSCs) and to investigate their proliferation and osteogenic differentiation on hydroxyapatite–collagen (HA–Col) scaffold. DPSCs were characterized by fluorescence-activated cell sorting (FACS). Cultured cells were CD73+, CD90+, CD105+ and CD31−, CD45−. A commercially available HA–Col scaffold was used for culture of DPSCs. Cell attachment and viability of DPSCs cultured on scaffold was studied by sulforhodamine assay. Osteoblast differentiation capacity was studied by alkaline phosphatase assay and the effects of growth factors such as PDGF, IGF1 and FGF2 were further studied. Scanning electron microscopy (SEM) of cell seeded scaffolds was also performed. We found that DPSCs cultured exhibited the characteristic mesenchymal stem cells (MSCs) morphology and differentiation properties. Scaffold was found to be non-cytotoxic and had good biocompatibility in vitro. Osteoblast differentiation ability was found to increase at higher concentration of scaffold and additive effects were observed with the use of growth factors. In SEM, cells appeared to cover the entire surface of the scaffold forming continuous cell layer and extending filopodial extensions. HA–Col scaffold is apt for MSCs attachment and proliferation in vitro. Their unique self-renewal and multilineage differential potential make them ideal for use in regenerative medicine. The limitations of currently available bone graft materials have led to the emergence of tissue engineering using mesenchymal stem cells (MSCs). Since, HA–Col scaffold potentiated the proliferation and osteogenic differentiation of DPSCs, this biomimetic material may be an ideal one for maxillofacial and alveolar bone regeneration.





Similar content being viewed by others
References
Chen Q, Shou P, Zheng C, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23(7):1128.
Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 2015;16(3):239–53.
Kassem M, Abdallah BM. Human bone-marrow-derived mesenchymal stem cells: biological characteristics and potential role in therapy of degenerative diseases. Cell Tissue Res. 2008;331(1):157–63.
Kim HJ, Park JS. Usage of human mesenchymal stem cells in cell-based therapy: advantages and disadvantages. Dev Reprod. 2017;21(1):1.
Garba A, Desmarets LM, Acar DD, et al. Immortalized porcine mesenchymal cells derived from nasal mucosa, lungs, lymph nodes, spleen and bone marrow retain their stemness properties and trigger the expression of siglec-1 in co-cultured blood monocytic cells. PLoS One. 2017;12(10):e0186343.
Nuti N, Corallo C, Chan BM, et al. Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev Rep. 2016;12(5):511–23.
Potdar PD, Jethmalani YD. Human dental pulp stem cells: applications in future regenerative medicine. World J Stem Cells. 2015;7(5):839.
Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1(3):1112–6.
Yang M, Arai A, Udagawa N, et al. Parathyroid hormone shifts cell fate of a leptin receptor-marked stromal population from adipogenic to osteoblastic lineage. J Bone Miner Res. 2019. https://doi.org/10.1002/jbmr.3811.
Gautam AK, Bhargavan B, Tyagi AM, et al. Differential effects of formononetin and cladrin on osteoblast function, peak bone mass achievement and bioavailability in rats. J Nutr Biochem. 2011;22(4):318–27.
Fotakis G, Timbrell JA. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 2006;160(2):171–7.
Reible B, Schmidmaier G, Moghaddam A, et al. Insulin like growth factor-1 as a possible alternative to bone morphogenetic protein-7 to induce osteogenic differentiation on human mesenchymal stem cells in vitro. Int J Mol Sci. 2018;19(6):1674.
Khan Y, Yaszemski MJ, Mikos AG, et al. Tissue engineering of bone: material and matrix considerations. J Bone Jt Surg. 2008;90:36–42.
Sancilio S, Gallorini M, Di Nisio C et al. Alginate/hydroxyapatite-based nanocomposite scaffolds for bone tissue engineering improve dental pulp biomineralization and differentiation. Stem Cells Int. 2018;2018:9643721. https://doi.org/10.1155/2018/9643721.
Masuda HT, Ishihara S, Harada I, et al. Coating extracellular matrix proteins on a (3-aminopropyl) triethoxysilanetreated glass substrate for improved cell culture. Biotechniques. 2014;56(4):172–9.
van den Dolder J, Jansen JA. The response of osteoblast-like cells towards collagen type I coating immobilized by p-nitrophenyl chloroformate to titanium. J Biomed Mater Res A. 2007;83(3):712–9.
Vandrovcová MA, Douglas TI, Hauk DO, et al. Influence of collagen and chondroitin sulfate (CS) coatings on poly-(lactide-co-glycolide) (PLGA) on MG 63 osteoblast-like cells. Physiol Res. 2011;60(5):797–813.
Zhang Y, Reddy VJ, Wong SY, et al. Enhanced biomineralization in osteoblasts on a novel electrospun biocomposite nanofibrous substrate of hydroxyapatite/collagen/chitosan. Tissue Eng Part A. 2010;16(6):1949–60.
Antebi B, Cheng X, Harris JN, et al. Biomimetic collagen–hydroxyapatite composite fabricated via a novel perfusion-flow mineralization technique. Tissue Eng Part C Methods. 2013;19(7):487–96.
Ning L, Malmström H, Ren YF. Porous collagen–hydroxyapatite scaffolds with mesenchymal stem cells for bone regeneration. J Oral Implantol. 2015;41(1):45–9.
Atari M, Caballé-Serrano J, Gil-Recio C, et al. The enhancement of osteogenesis through the use of dental pulp pluripotent stem cells in 3D. Bone. 2012;50(4):930–41.
Kashef-Saberi MS, Roodbari NH, Parivar K, et al. Enhanced osteogenic differentiation of mesenchymal stem cells on electrospun polyethersulfone/poly(vinyl) alcohol/platelet rich plasma nanofibrous scaffold. ASAIO J. 2018;4(5):e115–22.
Li A, Xia X, Yeh J, et al. PDGF-AA promotes osteogenic differentiation and migration of mesenchymal stem cell by down-regulating PDGFRα and derepressing BMP-Smad 1/5/8 signaling. PLoS One. 2014;9(12):e113785.
Kumar A, Salimath BP, Stark GB, et al. Platelet derived growth factor receptor signaling is not involved in osteogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2010;16(3):983–93.
Acknowledgements
We would like to thank Dr. Prabhaker Mishra, Department of Biostatistics and Health Informatics, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, for providing assistance in statistical analysis. This project was partially funded by the Department of Health Research, New Delhi, India (DHR-MRU 014).
Funding
No funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10266_2019_464_MOESM2_ESM.pdf
Supplementary material 2 Supplementary Fig. 1. After 10 days of osteogenic differentiation concentration of RANKL was determined by ELISA in cell lysate of DPMSCs grown with or without HA–collagen scaffold. Data are presented as mean ± SEM (n = 2). RANKL, Receptor Activator of Nuclear Factor Kappa. (PDF 5 kb)
Rights and permissions
About this article
Cite this article
Trivedi, S., Srivastava, K., Saluja, T.S. et al. Hydroxyapatite–collagen augments osteogenic differentiation of dental pulp stem cells. Odontology 108, 251–259 (2020). https://doi.org/10.1007/s10266-019-00464-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-019-00464-0