Abstract
Multicellularity arose several times in evolution of eukaryotes. The volvocine algae have full range of colonial organization from unicellular to colonies, and thus these algae are well-known models for examining the evolution and mechanisms of multicellularity. Gonium pectorale is a multicellular species of Volvocales and is thought to be one of the first small colonial organisms among the volvocine algae. In these algae, a cytoplasmic bridge is one of the key traits that arose during the evolution of multicellularity. Here, we observed the inversion process and the cytoplasmic bridges in G. pectorale using time-lapse, fluorescence, and electron microscopy. The cytoplasmic bridges were located in the middle region of the cell in 2-, 4-, 8-, and 16-celled stages and in inversion stages. However, there were no cytoplasmic bridges in the mature adult stage. Cytoplasmic bridges and cortical microtubules in G. pectorale suggest that a mechanism of kinesin-microtubule machinery similar to that in other volvocine algae is responsible for inversion in this species.






Similar content being viewed by others
References
Bisalputra T, Stein JR (1966) The development of cytoplasmic bridges in Volvox aureus. Can J Bot 44:1697–1702
Deason TR, Darden WH, Ely S (1969) The development of sperm packets of the M5 strain of Volvox aureus. J Ultrastruct Res 26:85–94
Fulton AB (1978) Colonial development in Pandorina morum. II. Colony morphogenesis and formation of the extracellular matrix. Dev Biol 64:236–251
Gerisch G (1959) Die Zelldifferenzierung bei Pleodorina californica Shaw und die Organisation der Phytomonadinekolonien. Arch Protistenkd 104:292–358
Green KJ, Kirk DL (1981) Cleavage patterns, cell lineages, and development of a cytoplasmic bridge system in Volvox embryos. J Cell Biol 91:743–755. doi:10.1083/jcb.91.3.743
Green KJ, Viamontes GI, Kirk DL (1981) Mechanism of formation, ultrastructure, and function of the cytoplasmic bridge system during morphogenesis in Volvox. J Cell Biol 91:756–769. doi:10.1083/jcb.91.3.756
Greuel BT, Floyd GL (1985) Development of the flagellar apparatus and flagellar orientation in the colonial green alga Gonium pectorale (Volvocales). J Phycol 21:358–371
Hallmann A (2006) Morphogenesis in the family Volvocaceae: different tactics for turning an embryo right-side out. Protist 157:445–461. doi:10.1016/j.protis.2006.05.010
Hallmann A (2011) Evolution of reproductive development in the volvocine algae. Sex Plant Reprod 24:97–112. doi:10.1007/s00497-010-0158-4
Harper RA (1912) The structure and development of the colony in Gonium. Trans Am Microsc Soc 31:65–83
Harris DO, Starr RC (1969) Life history and physiology of reproduction of Platydorina caudata Kofoid. Arch Protistenkd 111:138–155
Hartmann M (1924) Üeber die Veränderung der Koloniebildung von Eudorina elegans und Gonium pectorale unter dem Einfluss äuβerer Bedingungen. Mitt.der Untersuchungen über die Morphologie und Physiologie des Formwechsels der Phytomonadinen (Volvocales). Arch Protistenkd 49:375–395
Herron MD, Michod RE (2008) Evolution of complexity in the volvocine algae: transitions in individuality through Darwin’s eye. Evolution 62(2):436–451. doi:10.1111/j.1558-5646.2007.00304.x
Hoops HJ, Nishii I, Kirk DL (2005) Cytoplasmic bridges in Volvox and its relatives. In: Baluska F, Volkmann D, Barlow PW (eds) Cell–cell channels. Eurekah.com, Georgetown, TX, pp 1–20
Iida H, Nishii I, Inouye I (2011) Embryogenesis and cell positioning in Platydorina caudata (Volvocaceae, Chlorophyta). Phycologia 50:530–540. doi:10.2216/10-80.1
Kelland JL (1977) Inversion in Volvox (Chlorophyceae). J Phycol 13:373–378. doi:10.1111/j.1529-8817.1977.tb00614.x
Kirk DL (1998) Volvox: molecular-genetic origins of multicellularity and cellular differentiation. Developmental and cell biology series. Cambridge University Press, Cambridge
Kirk DL (2005) A twelve-step program for evolving multicellularity and a division of labor. BioEssays 27:299–310. doi:10.1002/bies.20197
Kirk DL, Kirk MM (1983) Protein synthetic patterns during the asexual life cycle of Volvox carteri. Dev Biol 96:493–506. doi:10.1016/0012-1606(83)90186-0
Kirk DL, Nishii I (2001) Volvox carteri as a model for studying the genetic and cytological control of morphogenesis. Dev Growth Differ 43:621–631
Kirk DL, Viamontes GI, Green KJ, Bryant JL Jr (1982) Integrated morphogenetic behavior of cell sheets: Volvox as a model. In: Subtelny S, Green PB (eds) Developmental order: Its Origin and Regulation. Alan R. Liss, New York, pp 247–274
Lang NJ (1963) Electron microscopy of the Volvocaceae and Astrephomaneceae. Am J Bot 50:280–300
Marchant HJ (1977) Colony formation and inversion in the green alga Eudorina elegans. Protoplasma 93:325–339
Meyer A (1896) Die Plasmaverbindung und die Membranen von Volvox. Bot Zeitg 54:187–217
Nishii I, Ogihara S (1999) Actomyosin contraction of the posterior hemisphere is required for inversion of the Volvox embryo. Development 126:2117–2127
Nishii I, Ogihara S, Kirk DL (2003) A kinesin, InvA, plays an essential role in Volvox morphogenesis. Cell 113:743–753. doi:10.1016/S0092-8674(03)00431-8
Nozaki H (2003) Origin and evolution of the genera Pleodorina and Volvox (Volvocales). Biologia (Bratislava) 58:425–431
Pickett-Heaps JD (1970) Some ultrastructural features of Volvox, with particular reference to the phenomenon of inversion. Planta 90:174–190
Pocock MA (1933) Volvox in South Africa. Ann S Afr Mus 16:523–646
Pocock MA (1955) Studies in North American Volvocales. I. The genus Gonium. Madroño 13:49–64
Stein JR (1958) A morphologic and genetic study of Gonium pectorale. Am J Bot 45:664–672
Stein JR (1965) On cytoplasmic strands in Gonium pectorale (Volvocales). J Phycol 1:1–5. doi:10.1111/j.1529-8817.1965.tb04547.x
Taft CE (1940) Asexual and sexual reproduction in Platydorina caudata Kofoid. Trans Am Microsc Soc 59:1–11
Taylor MG, Floyd GL, Hoops HJ (1985) Development of the flagellar apparatus and flagellar position in the colonial green alga Platydorina caudata (Chlorophyceae). J Phycol 21:533–546
Viamontes GI, Kirk DL (1977) Cell shape changes and the mechanism of inversion in Volvox. J Cell Biol 75:719–730
Viamontes GI, Fochtmann LJ, Kirk DL (1979) Morphogenesis in Volvox: analysis of critical variables. Cell 17:537–550. doi:10.1016/0092-8674(79)90262-9
Acknowledgments
We are grateful to Drs Ken-ichiro Ishida and Shinichi Miyamura (University of Tsukuba) for valuable advice during this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10265_2013_553_MOESM1_ESM.avi
Supplementary Figure S1. Microscopic time-lapse video of inversion in G. pectorale: Embryogenesis from 4-celled embryo to mature 8-celled colonies. (AVI 2179 kb)
10265_2013_553_MOESM2_ESM.tif
Supplementary Figure S2. a Merged image of DIC, microtubules (green), and EtBr-stained nuclei (red) corresponding to Fig. 5d-f. b, c Microtubules of the posterior region of the embryo are shown along the z-axis. Note that few cortical microtubules are localized in the posterior region of each cell. (TIFF 953 kb)
10265_2013_553_MOESM3_ESM.tif
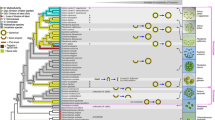
Supplementary Figure S3. Simplified schematic tree of the volvocine algae based on the phylogenetic analysis of Herron and Michod (2008). Black branches indicate multicellular species. Arrowhead indicates acquisition of cytoplasmic bridges in embryos. Double arrowhead indicates acquisition of complete inversion. Note that the genus Gonium is a member of the basal clade within Volvocales and incomplete inversion arose independently in the Gonium lineage. (TIFF 345 kb)
Rights and permissions
About this article
Cite this article
Iida, H., Ota, S. & Inouye, I. Cleavage, incomplete inversion, and cytoplasmic bridges in Gonium pectorale (Volvocales, Chlorophyta). J Plant Res 126, 699–707 (2013). https://doi.org/10.1007/s10265-013-0553-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-013-0553-7