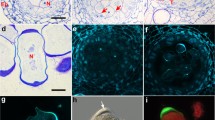
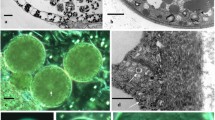
Abstract
As the earliest divergent land plants, bryophytes (mosses, hornworts, and liverworts) provide insight into the evolution of the unique plant process of sporogenesis by which meiosis results in heavy walled spores. New immunohistochemical data on microtubules and γ-tubulin in four genera of complex thalloid liverworts combined with previously published data on another four genera demonstrate grades in the evolution of spindle organization in meiosis. We have discovered that all recognized forms of microtubule organizing centers (MTOCs) in plant cells (plastid MTOCs, spheroid cytoplasmic MTOCs, polar organizers, and nuclear envelope MTOCs) occur in organization of the meiotic spindle of complex thalloid liverworts. In addition, all aspects of pre-meiotic preparation for quadripartitioning of the sporocyte into a tetrad of spores occur, with the exception of pre-meiotic wall precursors found in certain simple thalloids. The preparation includes morphogenetic plastid migration, cortical bands of microtubules that mark future cytokinetic planes in pre-meiosis, quadrilobing of the cytoplasm during meiotic prophase, and quadripolar microtubule systems that are transformed into functionally bipolar metaphase I spindles. Quadripolar spindle origin is typical of bryophyte sporogenesis even though the MTOCs involved may differ. However, in certain crown taxa of complex thalloids the spindle develops with no traces of quadripolarity and placement of intersporal walls is determined after meiosis, as is typical of higher plants.













Similar content being viewed by others

References
Brown RC, Lemmon BE (1988) Cytokinesis occurs at boundaries of domains delimited by nuclear based microtubules in sporocytes of Conocephalum conicum (Bryophyta). Cell Motil Cytoskelet 11:139–146
Brown RC, Lemmon BE (1995) Methods in plant immunolight microscopy. Meth Cell Biol 49:85–107
Brown RC, Lemmon BE (1997) The quadripolar microtubule system in lower land plants. J Plant Res 110:93–106
Brown RC, Lemmon BE (2001) The cytoskeleton and the spatial control of cytokinesis in the plant life cycle. Protoplasma 215:35–49
Brown RC, Lemmon BE (2004) γ-Tubulin, microtubule arrays, and quadripolarity during sporogenesis in the Hepatic Aneura pinguis (L.) Dumort. (Metzgeriales). J Plant Res 117:371–376
Brown RC, Lemmon BE (2006) Polar organizers and girdling bands of microtubules are associated with γ-tubulin and act in establishment of meiotic quadripolarity in the hepatic Aneura pinguis (Bryophyta). Protoplasma 227:77–85
Brown RC, Lemmon BE (2007) The pleiomorphic plant MTOC: an evolutionary perspective. J Int Plant Biol 49:1142–1153
Brown RC, Lemmon BE (2008) γ-Tubulin and microtubule organization during meiosis in the liverwort Ricciocarpus natans (Ricciaceae). Am J Bot 95:664–671
Brown RC, Lemmon BE (2009) Pre-meiotic bands and novel meiotic spindle ontogeny in quadrilobed sporocytes of leafy liverworts (Jungermannidae, Bryophyta). Protoplasma. doi:10.1007/s00709-009-0073-4
Brown RC, Lemmon BE, Renzaglia KS (1986) Sporocytic control of spore wall pattern in liverworts. Am J Bot 73:593–596
Brown RC, Lemmon BE, Horio T (2004) γ-Tubulin localization changes from discrete polar organizers to anastral spindles and phragmoplasts in mitosis of Marchantia polymorpha L. Protoplasma 224:187–193
Brown RC, Lemmon BE, Shimamura M (2007) Transformations of the pleiomorphic plant MTOC during sporogenesis in the hepatic Marchantia polymorpha L. J Int Plant Biol 49:1244–1252
Carafa A, Duckett JG, Ligrone R (2003) The placenta in Monoclea fosteri Hook. and Treubia lacunosa (Col.) Prosk.: insights into placental evolution in liverworts. Ann Bot 92:299–307
Crandall-Stotler B, Stotler RE (2000) Morphology and classification of the Marchantiophyta. In: Shaw AJ, Goffinet B (eds) Bryophyte biology. Cambridge University Press, Cambridge, pp 21–70
Crandall-Stotler B, Stotler RE, Long DG (2008) Morphology and classification of the Marchantiophyta. In: Goffinet B, Shaw AJ (eds) Bryophyte biology, 2nd edn. Cambridge University Press, Cambridge, pp 1–54
Crandall-Stotler B, Stotler RE, Long DG (2009) Morphology and classification of the Marchantiophyta. Edinb J Bot 66:155–198
Farmer JB (1895) On spore-formation and nuclear division in the Hepaticae. Ann Bot 9:469–523
Forrest LL, Davis EC, Long DG, Crandall-Stotler BJ, Clark A, Hollingsworth ML (2006) Unraveling the evolutionary history of the liverworts (Marchantiophyta): multiple taxa, genomes and analyses. Bryologist 109:303–334
Frey W, Stech M (2005) A morpho-molecular classification of the liverworts (Hepaticophytina, Bryophyta). Nova Hedw 81:55–78
Horio T, Basaki A, Takeoka A, Yamato M (1999) Lethal level overexpression of γ-tubulin in fission yeast causes mitotic arrest. Cell Motil Cytoskelet 44:284–295
Long DG (2006) New higher taxa of complex thalloid liverworts (Marchantiophyta-Marchantiopsida). Edinb J Bot 63:257–262
Mineyuki Y (1999) The preprophase band of microtubules: its function as a cytokinetic apparatus in higher plants. Int Rev Cytol 187:1–49
Mineyuki Y (2007) Plant microtubule studies: past and present. J Plant Res 120:45–51
Neidhart HV (1978) Ultrastructural aspects of sporogenesis in Riella affinis Howe and Underwood (Hepaticae). J Bryol 10:145–154
Ovenchkina Y, Oakley BR (2001) γ-Tubulin in plant cells. Methods Cell Biol 67:195–212
Renzaglia KS, Brown RC, Lemmon BE, Duckett JG, Ligrone R (1994) Occurrence and phylogenetic significance of monoplastidic meiosis in liverworts. Can J Bot 72:65–72
Renzaglia KS, Schuette S, Duff RJ, Ligrone R, Shaw AJ, Mishler BD, Duckett JG (2007) Bryophyte phylogeny: advancing the molecular and morphological frontiers. Bryologist 110:179–213
Schmit A-C (2002) Acentrosomal microtubule nucleation in higher plants. Int Rev Cytol 220:257–289
Schuster RM (1984) Evolution, phylogeny and classification of the Hepaticae. In: Schuster RM (ed) New manual of bryology, vol 2. Hattori Botanical Laboratory, Nichinan, pp 892–1007
Schuster RM (1992) The Hepaticae and Anthocerotae of North America east of the hundredth meridian, vol VI. Field Museum of Natural History, Chicago
Shaw J, Renzaglia K (2004) Phylogeny and diversification of bryophytes. Am J Bot 91:1557–1581
Shimamura M, Deguchi H, Mineyuki Y (1998) Meiotic cytokinetic apparatus in the formation of the linear spore tetrads of Conocephalum japonicum (Bryophyta). Planta 206:604–610
Shimamura M, Mineyuki Y, Deguchi H (2000) Monoplastidic meiosis in Dumortiera hirsuta (Bryophyta; Marchantiales). J Hattori Bot Lab 88:267–270
Shimamura M, Kitamura A, Mineyuki Y, Deguchi H (2001) Occurrence of monoplastidic sporocytes and quadripolar microtubule systems in Marchantiales (Bryophyta; Marchantiidae). Hikobia 13:551–562
Shimamura M, Deguchi H, Mineyuki Y (2003) A review of the occurrence of monoplastidic meiosis in liverworts. J Hattori Bot Lab 94:179–186
Shimamura M, Brown RC, Lemmon BE, Akashi T, Mizuno K, Nishihara N et al (2004) γ-Tubulin in basal land plants: characterization, localization and implication in the evolution of acentriolar microtubule organizing centers. Plant Cell 16:45–59
Shimamura M, Furuki T, Deguchi H (2005) Sporophyte anatomy of Cavicularia densa (Blasiaceae). Bryologist 108:420–426
Stoppin VM, Vantard M, Schmit A-C, Lambert A-M (1994) Isolated plant nuclei nucleate microtubule assembly: the nuclear surface in higher plants has centrosome-like activity. Plant Cell 6:1099–1106
Vaughn KC, Harper JD (1998) Microtubule-organizing centers and nucleating sites in land plants. Int Rev Cytol 181:75–149
Wasteneys GO (2002) Microtubule organization in the green kingdom: chaos or self-order? J Cell Sci 115:1345–1354
Wiese C, Zheng Y (2006) Microtubule nucleation: γ-tubulin and beyond. J Cell Sci 119:4143–4153
Acknowledgments
We thank Dr Tetsuya Horio (University of Kansas) for the gift of the G9 antibody, Dr Suzanne Fredericq (University of Louisiana) for help with phylogenetics, and Dr Paul Davison (University of North Alabama) for help with taxonomic identifications and for collections of Blasia pusilla.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brown, R.C., Lemmon, B.E. & Shimamura, M. Diversity in meiotic spindle origin and determination of cytokinetic planes in sporogenesis of complex thalloid liverworts (Marchantiopsida). J Plant Res 123, 589–605 (2010). https://doi.org/10.1007/s10265-009-0286-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-009-0286-9