Abstract
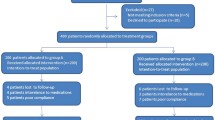
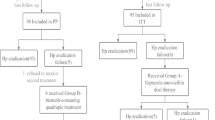
To evaluate potency and safety of 14-day bismuth–furazolidone quadruple regimens and to compare efficacies of five proton pump inhibitors (PPIs) for the initial eradication of Helicobacter pylori (H. pylori), 175 eligible patients were enrolled and randomly assigned to 14-day quadruple regimens consisting of bismuth (400 mg), amoxicillin (1 g), furazolidone (100 mg), and a PPI, twice a day. PPIs used were Group A (pantoprazole capsules, 40 mg), Group B (pantoprazole tablets, 40 mg), Group C (lansoprazole, 30 mg), Group D (esomeprazole, 20 mg), and Group E (rabeprazole, 10 mg). H. pylori status was reassessed by 13C urea breath test on day 56 as the primary outcome. Gastrointestinal symptoms, parenteral side effects, compliance, and stool type were recorded simultaneously. The total eradication rates were 86.9% (152/175 [95% CI 80.9–91.5%]) and 95.6% (152/159 [91.1–98.2%]) by intention-to-treat (ITT) and per-protocol (PP) analysis. The efficacies of Group A, B, C, D, and E by ITT analysis were 91.4% (32/35 [76.9–98.2%]), 85.7% (30/35 [69.7–95.2%]), 88.6% (31/35 [73.3–96.8%]), 85.7% (30/35 [69.7–95.2%]), and 82.9% (29/35 [66.4–93.4%]) (p > 0.05). In the PP analysis, the efficacies were 97.0% (32/33), 93.8% (30/32), 93.9% (31/33), 100% (30/30), and 93.5% (29/31) (p > 0.05). Gastrointestinal symptoms and stool type were improved significantly (p < 0.05). Total side effects rate and poor compliance rate were 15.7% (25/159) and 5.0% (8/159). Fourteen-day bismuth–furazolidone quadruple regimens are of high potency and safety for the initial eradication of H. pylori. Efficacies of different PPIs and different dosages (9–32 mg omeprazole equivalents) showed no significant difference. The appropriate PPI can thus be chosen by clinicians.


Similar content being viewed by others
Abbreviations
- H. pylori :
-
Helicobacter pylori
- PPI:
-
Proton pump inhibitor
- GSRS:
-
Gastrointestinal symptom rating scale
- BSS:
-
Bristol stool scale
- ITT:
-
Intention-to-treat
- PP:
-
Per-protocol
- BMI:
-
Body mass index
- b.i.d:
-
Bis in diē/twice a day
- t.i.d:
-
Ter in diē/three times a day
- q.i.d:
-
Quater in diē/four times a day
References
Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212–39. https://doi.org/10.1038/ajg.2016.563.
Cave DR. Transmission and epidemiology of Helicobacter pylori. Am J Med. 1996;100(5A):12S–8S Discussion 7S-8S.
Nagy P, Johansson S, Molloy-Bland M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. 2016;8:8. https://doi.org/10.1186/s13099-016-0091-7.
Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–67. https://doi.org/10.1136/gutjnl-2015-309252.
Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017;66(1):6–30. https://doi.org/10.1136/gutjnl-2016-312288.
Chinese Society of G, Chinese Study Group on Helicobacter p, Peptic U, Liu G, Xie J, Lu ZR, et al. Fifth Chinese national consensus report on the management of Helicobacter pylori infection. Zhonghua Nei Ke Za Zhi. 2017;56(7):532–45. https://doi.org/10.3760/cma.j.issn.0578-1426.2017.07.014.
Kuo CH, Lu CY, Shih HY, Liu CJ, Wu MC, Hu HM, et al. CYP2C19 polymorphism influences Helicobacter pylori eradication. World J Gastroenterol. 2014;20(43):16029–36. https://doi.org/10.3748/wjg.v20.i43.16029.
Su P, Li Y, Li H, Zhang J, Lin L, Wang Q, et al. Antibiotic resistance of Helicobacter pylori isolated in the Southeast Coastal Region of China. Helicobacter. 2013;18(4):274–9. https://doi.org/10.1111/hel.12046.
Liu DS, Wang YH, Zeng ZR, Zhang ZY, Lu H, Xu JM, et al. Primary antibiotic resistance of Helicobacter pylori in Chinese patients: a multi-region prospective 7-year study. Clin Microbiol Infect. 2017. https://doi.org/10.1016/j.cmi.2017.11.010.
Graham DY, Lu H. Furazolidone in Helicobacter pylori therapy: misunderstood and often unfairly maligned drug told in a story of French bread. Saudi J Gastroenterol. 2012;18(1):1–2. https://doi.org/10.4103/1319-3767.91724.
Lu H, Zhang W, Graham DY. Bismuth-containing quadruple therapy for Helicobacter pylori: lessons from China. Eur J Gastroenterol Hepatol. 2013;25(10):1134–40. https://doi.org/10.1097/MEG.0b013e3283633b57.
Mohammadi M, Attaran B, Malekzadeh R, Graham DY. Furazolidone, an underutilized drug for H. pylori eradication: lessons from Iran. Dig Dis Sci. 2017;62(8):1890–6. https://doi.org/10.1007/s10620-017-4628-5.
Catalano F, Terminella C, Branciforte G, Bentivegna C, Brogna A, Scalia A. Eradication therapy with rabeprazole versus omeprazole in the treatment of active duodenal ulcer. Digestion. 2002;66(3):154–9. https://doi.org/10.1159/000066756.
Choi HS, Park DI, Hwang SJ, Park JS, Kim HJ, Cho YK, et al. Double-dose, new-generation proton pump inhibitors do not improve Helicobacter pylori eradication rate. Helicobacter. 2007;12(6):638–42. https://doi.org/10.1111/j.1523-5378.2007.00556.x.
Lee VW, Chau TS, Chan AK, Lee KK, Waye MM, Ling TK, et al. Pharmacogenetics of esomeprazole or rabeprazole-based triple therapy in Helicobacter pylori eradication in Hong Kong non-ulcer dyspepsia Chinese subjects. J Clin Pharm Ther. 2010;35(3):343–50. https://doi.org/10.1111/j.1365-2710.2009.01088.x.
Pan X, Li Y, Qiu Y, Tang Q, Qian B, Yao L, et al. Efficacy and tolerability of first-line triple therapy with levofloxacin and amoxicillin plus esomeprazole or rabeprazole for the eradication of Helicobacter pylori infection and the effect of CYP2C19 genotype: a 1-week, randomized, open-label study in Chinese adults. Clin Ther. 2010;32(12):2003–11. https://doi.org/10.1016/j.clinthera.2010.11.005.
Keum B, Lee SW, Kim SY, Kim JM, Choung RS, Yim HJ, et al. Comparison of Helicobacter pylori eradication rate according to different PPI-based triple therapy–omeprazole, rabeprazole, esomeprazole and lansoprazole. Korean J Gastroenterol = Taehan Sohwagi Hakhoe chi. 2005;46(6):433–9.
Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2017. https://doi.org/10.1016/j.cgh.2017.09.033.
Kirchheiner J, Glatt S, Fuhr U, Klotz U, Meineke I, Seufferlein T, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65(1):19–31. https://doi.org/10.1007/s00228-008-0576-5.
Chuah YY, Wu DC, Chuah SK, Yang JC, Lee TH, Yeh HZ, et al. Real-world practice and expectation of Asia-Pacific physicians and patients in Helicobacter pylori eradication (REAP-HP survey). Helicobacter. 2017. https://doi.org/10.1111/hel.12380.
Shao Y, Lu R, Yang Y, Xu Q, Wang B, Ye G. Antibiotic resistance of Helicobacter pylori to 16 antibiotics in clinical patients. J Clin Lab Anal. 2017. https://doi.org/10.1002/jcla.22339.
Liang X, Xu X, Zheng Q, Zhang W, Sun Q, Liu W, et al. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin Gastroenterol Hepatol. 2013;11(7):802–7. https://doi.org/10.1016/j.cgh.2013.01.008.
Nie Y, Li Y, Wu H, Sha W, Du H, Dai S, et al. Colloidal bismuth pectin: an alternative to bismuth subcitrate for the treatment of Helicobacter pylori–positive duodenal ulcer. Helicobacter. 1999;4(2):128–34.
Dore MP, Farina V, Cuccu M, Mameli L, Massarelli G, Graham DY. Twice-a-day bismuth-containing quadruple therapy for Helicobacter pylori eradication: a randomized trial of 10 and 14 days. Helicobacter. 2011;16(4):295–300. https://doi.org/10.1111/j.1523-5378.2011.00857.x.
Lu Z, Xie Y, Lu N, Zhou H, Liu Z, Zhu X, et al. Efficacy of triple versus quadruple furazolidone-based eradication regimens for Helicobacter pylori infection. Zhonghua Yi Xue Za Zhi. 2014;94(8):572–5.
Labenz J. Current role of acid suppressants in Helicobacter pylori eradication therapy. Best Pract Res Clin Gastroenterol. 2001;15(3):413–31. https://doi.org/10.1053/bega.2001.0188.
Kita T, Tanigawara Y, Aoyama N, Hohda T, Saijoh Y, Komada F, et al. CYP2C19 genotype related effect of omeprazole on intragastric pH and antimicrobial stability. Pharm Res. 2001;18(5):615–21.
Gisbert JP. Potent gastric acid inhibition in Helicobacter pylori eradication. Drugs. 2005;65(Suppl 1):83–96.
Calvet X, Gomollon F. What is potent acid inhibition, and how can it be achieved? Drugs. 2005;65(Suppl 1):13–23.
Sahara S, Sugimoto M, Uotani T, Ichikawa H, Yamade M, Iwaizumi M, et al. Twice-daily dosing of esomeprazole effectively inhibits acid secretion in CYP2C19 rapid metabolisers compared with twice-daily omeprazole, rabeprazole or lansoprazole. Aliment Pharmacol Ther. 2013;38(9):1129–37. https://doi.org/10.1111/apt.12492.
Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12(2):177–86. https://doi.org/10.1016/j.cgh.2013.05.028 Discussion e12-3.
Liu MK, Wu IC, Lu CY, Kuo CH, Yu FJ, Liu CJ, et al. Randomized trial comparing rabeprazole- versus lansoprazole-based Helicobacter pylori eradication regimens. Kaohsiung J Med Sci. 2013;29(7):379–84. https://doi.org/10.1016/j.kjms.2012.11.006.
Sarikaya M, Dogan Z, Ergul B, Filik L. Functional dyspepsia symptom resolution after Helicobacter pylori eradication with two different regimens. Prz Gastroenterol. 2014;9(1):49–52. https://doi.org/10.5114/pg.2014.40851.
Mokhtare M, Hosseini V, Tirgar Fakheri H, Maleki I, Taghvaei T, Valizadeh SM, et al. Comparison of quadruple and triple Furazolidone containing regimens on eradication of Helicobacter pylori. Med J Islam Repub Iran. 2015;29:195.
Xie Y, Zhu Y, Zhou H, Lu ZF, Yang Z, Shu X, et al. Furazolidone-based triple and quadruple eradication therapy for Helicobacter pylori infection. World J Gastroenterol. 2014;20(32):11415–21. https://doi.org/10.3748/wjg.v20.i32.11415.
Acknowledgements
We thank our patients and colleagues at Sir Run Run Shaw Hospital, Zhejiang University. This study was funded by Zhejiang Province Key Science and Technology Innovation Team (2013TD13), Science Foundation of Zhejiang Traditional Medicine Bureau (2012ZB098, 2017ZB063) and Natural Science Foundation of Zhejiang Province (LY18H160011).
Author information
Authors and Affiliations
Contributions
SC and AL contributed to the study design. LC and JH organized the study, enrolled participants and performed the writing of the manuscript. LW and QG performed the data entry and statistical analysis. XC, YL, YD and HH performed the preliminary screening of participants. HC and YC conducted the 13C urea breath test. All authors have participated in the discussion of the manuscript and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethics approval
This study was approved by the Ethics Committee of Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University (20161206-21).
Informed consent
Written informed consent was obtained from all participants before enrollment.
Additional information
Luyi Chen and Jiamin He have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, L., He, J., Wang, L. et al. Efficacies of different proton pump inhibitor-based 14-day bismuth–furazolidone quadruple regimens for the initial eradication of Helicobacter pylori in the southeast coastal region of China: an open-label, randomized clinical trial. Clin Exp Med 18, 569–576 (2018). https://doi.org/10.1007/s10238-018-0510-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-018-0510-9