Abstract
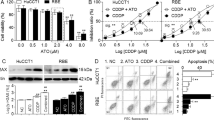
The purpose of our study was to investigate anticancer activity of arsenic trioxide (As2O3) on cholangiocarcinoma through in vitro and in vivo experiments using human cholangiocarcinoma cancer cells (CC-t6 cells) and a nude mouse model. The effect of As2O3 on CC-t6 cell survival was determined in vitro using MTT assay. Analysis of cell cycle phase distribution and quantification of apoptosis/necrosis, which were achieved by flow cytometry, were performed in order to understand the mechanism of As2O3. In vivo experiment was performed to assess the effectiveness of local injection of As2O3 on tumor inhibition by comparing the following three groups each consisting of five nude mouse xenograft models: high dose As2O3 (5 mg/kg), low dose As2O3 (1 mg/kg), and saline. In MTT assay, As2O3 inhibited the growth of CC-t6 cells more effectively than cisplatin or adriamycin at concentrations between 1 and 100 μM for most time points between 24 and 72 h (p < 0.05). With increased concentration of As2O3, there was dose-dependent increase in G 0/G 1 phase and dose-dependent decrease in S phase. As2O3-mediated inhibition of cell viability was achieved via induction of apoptosis and necrosis in a dose-dependent manner. Injection of As2O3 into CC-t6-induced tumors in nude mice inhibited the growth of subcutaneous tumor xenografts. As2O3 treatment dose-dependently inhibited the proliferation of CC-t6 cells via G 0/G 1 phase arrest and retarded tumor growth in nude mice, suggesting that As2O3 may be effective in the treatment of cholangiocarcinoma.







Similar content being viewed by others
References
Burger I, Hong K, Schulick R, Georgiades C, Thuluvath P, Choti M, Kamel I, Geschwind JF (2005) Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol 16:353–361
Shaib YH, El-Serag HB, Nooka AK, Thomas M, Brown TD, Patt YZ, Hassan MM (2007) Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol 102:1016–1021
Primary liver cancer in Japan (1990) Clinicopathologic features and results of surgical treatment. Liver Cancer Study Group of Japan. Ann Surg 211:277–287
The Statistics of Cancer Registry (2008) The annual report of national cancer registry. Ministry of Health and Welfare/National cancer center, Korea
Patel T (2001) Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 33:1353–1357
Hammill CW, Wong LL (2008) Intrahepatic cholangiocarcinoma: a malignancy of increasing importance. J Am Coll Surg 207:594–603
Madariaga JR, Iwatsuki S, Todo S, Lee RG, Irish W, Starzl TE (1998) Liver resection for hilar and peripheral cholangiocarcinomas: a study of 62 cases. Ann Surg 227:70–79
Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, Yoshidome H, Miyazaki M (2002) Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg 89:1525–1531
Kim JH, Yoon HK, Sung KB, Ko GY, Gwon DI, Shin JH, Song HY (2008) Transcatheter arterial chemoembolization or chemoinfusion for unresectable intrahepatic cholangiocarcinoma: clinical efficacy and factors influencing outcomes. Cancer 113:1614–1622
Antman KH (2001) Introduction: the history of arsenic trioxide in cancer therapy. Oncologist 6(Suppl 2):1–2
Kwong YL, Todd D (1997) Delicious poison: arsenic trioxide for the treatment of leukemia. Blood 89:3487–3488
Zhang P, Wang S, Hu L, Qiu F, Yang H, Xiao Y, Li X, Han X, Zhou J, Liu P (2000) Seven years’ summary report on the treatment of acute promyelocytic leukemia with arsenic trioxide–an analysis of 242 cases. Zhonghua Xue Ye Xue Za Zhi 21:67–70
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY (1997) Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood 89:3354–3360
Murgo AJ (2001) Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the National Cancer Institute Cooperative Research and Development Studies. Oncologist 6(Suppl 2):22–28
Zheng J, Deng YP, Lin C, Fu M, Xiao PG, Wu M (1999) Arsenic trioxide induces apoptosis of HPV16 DNA-immortalized human cervical epithelial cells and selectively inhibits viral gene expression. Int J Cancer 82:286–292
Shen ZY, Zhang Y, Chen JY, Chen MH, Shen J, Luo WH, Zeng Y (2004) Intratumoral injection of arsenic to enhance antitumor efficacy in human esophageal carcinoma cell xenografts. Oncol Rep 11:155–159
Zhong F, Zhang S, Shao C, Yang J, Wu X (2010) Arsenic trioxide inhibits cholangiocarcinoma cell growth and induces apoptosis. Pathol Oncol Res 16:413–420
Institute of Laboratory Animal Research (1996) Commission on life sciences, national research council. National Academy Press, Washington
Krishan A (1975) Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol 66:188–193
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C (1995) A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 184:39–51
Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RP Jr (1998) Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med 339:1341–1348
Charoentum C, Thongprasert S, Chewaskulyong B, Munprakan S (2007) Experience with gemcitabine and cisplatin in the therapy of inoperable and metastatic cholangiocarcinoma. World J Gastroenterol 13:2852–2854
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362(14):1273–1281
Kirchhoff T, Zender L, Merkesdal S, Frericks B, Malek N, Bleck J, Kubicka S, Baus S, Chavan A, Manns MP, Galanski M (2005) Initial experience from a combination of systemic and regional chemotherapy in the treatment of patients with nonresectable cholangiocellular carcinoma in the liver. World J Gastroenterol 11:1091–1095
Park SH, Park YH, Lee JN, Bang SM, Cho EK, Shin DB, Lee JH (2006) Phase II study of epirubicin, cisplatin, and capecitabine for advanced biliary tract adenocarcinoma. Cancer 106:361–365
Kito M, Akao Y, Ohishi N, Yagi K, Nozawa Y (2002) Arsenic trioxide-induced apoptosis and its enhancement by buthionine sulfoximine in hepatocellular carcinoma cell lines. Biochem Biophys Res Commun 291:861–867
Wang ZG, Rivi R, Delva L, Konig A, Scheinberg DA, Gambacorti-Passerini C, Gabrilove JL, Warrell RP Jr, Pandolfi PP (1998) Arsenic trioxide and melarsoprol induce programmed cell death in myeloid leukemia cell lines and function in a PML and PML-RARalpha independent manner. Blood 92:1497–1504
Maeda H, Hori S, Nishitoh H, Ichijo H, Ogawa O, Kakehi Y, Kakizuka A (2001) Tumor growth inhibition by arsenic trioxide (As2O3) in the orthotopic metastasis model of androgen-independent prostate cancer. Cancer Res 61:5432–5440
Ora I, Bondesson L, Jonsson C, Ljungberg J, Porn-Ares I, Garwicz S, Pahlman S (2000) Arsenic trioxide inhibits neuroblastoma growth in vivo and promotes apoptotic cell death in vitro. Biochem Biophys Res Commun 277:179–185
Xiao YF, Wu DD, Liu SX, Chen X, Ren LF (2007) Effect of arsenic trioxide on vascular endothelial cell proliferation and expression of vascular endothelial growth factor receptors Flt-1 and KDR in gastric cancer in nude mice. World J Gastroenterol 13:6498–6505
Miller WH Jr, Schipper HM, Lee JS, Singer J, Waxman S (2002) Mechanisms of action of arsenic trioxide. Cancer Res 62:3893–3903
Nakagawa Y, Akao Y, Morikawa H, Hirata I, Katsu K, Naoe T, Ohishi N, Yagi K (2002) Arsenic trioxide-induced apoptosis through oxidative stress in cells of colon cancer cell lines. Life Sci 70:2253–2269
Dai J, Weinberg RS, Waxman S, Jing Y (1999) Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood 93:268–277
Chen YC, Lin-Shiau SY, Lin JK (1998) Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol 177:324–333
Kim HJ, Shin JH, Kim TH, Kim EY, Park YS, Park CS, Song HY (2009) Efficacy of transarterial embolization with arsenic trioxide oil emulsion in a rabbit VX2 liver tumor model. J Vasc Interv Radiol 20:1365–1370
Huang SY, Chang CS, Tang JL, Tien HF, Kuo TL, Huang SF, Yao YT, Chou WC, Chung CY, Wang CH, Shen MC, Chen YC (1998) Acute and chronic arsenic poisoning associated with treatment of acute promyelocytic leukaemia. Br J Haematol 103:1092–1095
Acknowledgments
This work was supported by a National Research Foundation of Korea Grant from the Korean Government (KRF-2006-331-E00111). This study was supported by a grant (2011-312) from the Asan Institute for Life Sciences, Seoul, Korea.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Eun-Young Kim and Sang Soo Lee contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kim, EY., Lee, S.S., Shin, J.H. et al. Anticancer effect of arsenic trioxide on cholangiocarcinoma: in vitro experiments and in vivo xenograft mouse model. Clin Exp Med 14, 215–224 (2014). https://doi.org/10.1007/s10238-013-0233-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-013-0233-x