Abstract
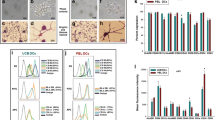
Allogeneic cord blood transplantation is associated with a less severe graft-versus-host disease (GVHD). This observation is thought to be due to immaturity of cord blood cell immune capabilities. Dendritic cells (DCs) are the most potent antigen-presenting cells of the immune system capable of initiation and regulation of immune responses. In this investigation, we hypothesized that non-manipulated cord blood dendritic cells (CBDCs) not only differ in their functional maturity from adult peripheral blood DCs (PBDCs) but also differ in their subsets and their preference in promoting Th1 or Th2 immune responses. Non-manipulated fresh DCs were isolated from cord blood (CB) and adult peripheral blood (PB) mononuclear cells as lineage marker negative cells. The differences in expression of costimulatory molecules, the proportion of myeloid and lymphoid DCs subsets, their immunostimulatory characteristics and their influence on promoting the differentiation of naïve T cells towards Th1 or Th2 cells were then investigated in these two populations. Our results showed that freshly isolated CBDCs, similar to cord blood monocyte derived DCs, were poor inducers of IFN-γ secretion while they increased the induction of IL-4 production by T cells in comparison with PBDCs. CBDCs were also poor stimulators of allogenic T cells in mixed leukocyte reaction compared to adult peripheral blood dendritic cells. They also displayed decreased expression of HLA-DR and CD86 molecules. The ratio of lymphoid DCs (CD11c−, CD123+) to myeloid DCs (CD11c+, CD123−) was significantly higher in CB compared to PB. We conclude that CBDCs preferential priming of naive T cells towards Th2 population, seems to be an intrinsic property independent of their subtype. This property along with their functional immaturity should contribute to outcome of cord blood transplantation.


Similar content being viewed by others
References
Rocha V, Wagner JE Jr, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM et al (2000) Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med 342:1846–1854
Teshima T, Ferrara JL (2002) Understanding the alloresponse: new approaches to graft-versus-host disease prevention. Semin Hematol 39:15–22
Yamazaki S, Inaba K, Tarbell KV, Steinman RM (2006) Dendritic cells expand antigen-specific Foxp3+CD25+CD4+regulatory T cells including suppressors of alloreactivity. Immunol Rev 212:314–329
Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J et al (1999) Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science 285:412–415
Ferrara JL, Cooke KR, Pan L, Krenger W (1996) The immunopathophysiology of acute graft-versus-host-disease. Stem Cells 14:473–489
Hart DN (1997) Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 90:3245–3287
Steinman RM (1991) The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 9:271–296
Fazekas de St Groth B (1998) The evolution of self-tolerance: a new cell arises to meet the challenge of self-reactivity. Immunol Today 19:448–454
Steptoe RJ, Thomson AW (1996) Dendritic cells and tolerance induction. Clin Exp Immunol 105:397–402
Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S et al (2000) BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol 165:6037–6046
O’Doherty U, Steinman RM, Peng M, Cameron PU, Gezelter S, Kopeloff I et al (1993) Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J Exp Med 178:1067–1076
Thomas R, Davis LS, Lipsky PE (1993) Isolation and characterization of human peripheral blood dendritic cells. J Immunol 150:821–834
Almeida J, Bueno C, Alguero MC, Sanchez ML, Canizo MC, Fernandez ME et al (1999) Extensive characterization of the immunophenotype and pattern of cytokine production by distinct subpopulations of normal human peripheral blood MHC II +/lineage- cells. Clin Exp Immunol 118:392–401
Almeida J, Bueno C, Alguero MC, Sanchez ML, de Santiago M, Escribano L et al (2001) Comparative analysis of the morphological, cytochemical, immunophenotypical, and functional characteristics of normal human peripheral blood lineage(-)/CD16(+)/HLA-DR(+)/CD14(-/lo) cells, CD14(+) monocytes, and CD16(-) dendritic cells. Clin Immunol 100:325–338
Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ et al (2000) Immunobiology of dendritic cells. Annu Rev Immunol 18:767–811
Lechler R, Ng WF, Steinman RM (2001) Dendritic cells in transplantation–friend or foe? Immunity 14:357–368
Steinman RM, Nussenzweig MC (2002) Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA 99:351–358
McLellan AD, Starling GC, Williams LA, Hock BD, Hart DN (1995) Activation of human peripheral blood dendritic cells induces the CD86 co-stimulatory molecule. Eur J Immunol 25:2064–2068
Hunt DW, Huppertz HI, Jiang HJ, Petty RE (1994) Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood 84:4333–4343
Petty RE, Hunt DW (1998) Neonatal dendritic cells. Vaccine 16:1378–1382
Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R et al (1999) Reciprocal control of T helper cell and dendritic cell differentiation. Science 283:1183–1186
Shortman K, Liu YJ (2002) Mouse and human dendritic cell subtypes. Nat Rev Immunol 2:151–161
Sorg RV, Kogler G, Wernet P (1999) Identification of cord blood dendritic cells as an immature CD11c-population. Blood 93:2302–2307
Borras FE, Matthews NC, Lowdell MW, Navarrete CV (2001) Identification of both myeloid CD11c + and lymphoid CD11c-dendritic cell subsets in cord blood. Br J Haematol 113:925–931
Moser M, Murphy KM (2000) Dendritic cell regulation of TH1–TH2 development. Nat Immunol 1:199–205
Yamaguchi N, Fujimori Y, Fujibayashi Y, Kasumoto I, Okamura H, Nakanishi K, Hara H (2005) Interferon-gamma production by human cord blood monocyte-derived dendritic cells. Ann Hematol 84:423–428
Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D (2002) Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol 128:118–123
Goriely S, Vincart B, Stordeur P, Vekemans J, Willems F, Goldman M et al (2001) Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol 166:2141–2146
Liu E, Tu W, Law HK, Lau YL (2001) Decreased yield, phenotypic expression and function of immature monocyte-derived dendritic cells in cord blood. Br J Haematol 113:240–246
McLellan AD, Sorg RV, Williams LA, Hart DN (1996) Human dendritic cells activate T lymphocytes via a CD40: CD40 ligand-dependent pathway. Eur J Immunol 26:1204–1210
Darmochwal-Kolarz D, Rolinski J, Buczkowski J, Tabarkiewicz J, Leszczynska-Gorzelak B, Zych I et al (2004) CD1c(+) immature myeloid dendritic cells are predominant in cord blood of healthy neonates. Immunol Lett 91:71–74
Hausser G, Ludewig B, Gelderblom HR, Tsunetsugu-Yokota Y, Akagawa K, Meyerhans A (1997) Monocyte-derived dendritic cells represent a transient stage of differentiation in the myeloid lineage. Immunobiology 197:534–542
Vanden Eijnden S, Goriely S, De Wit D, Goldman M, Willems F (2006) Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur J Immunol 36:21–26
Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA et al (1995) Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4 + T cells. J Immunol 154:5071–5079
Trinchieri G (1995) Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol 13:251–276
Arulanandam BP, Van Cleave VH, Metzger DW (1999) IL-12 is a potent neonatal vaccine adjuvant. Eur J Immunol 29:256–264
Donckier V, Flamand V, Desalle F, Vanderhaeghen ML, de Veerman M, Thielemans K et al (1998) IL-12 prevents neonatal induction of transplantation tolerance in mice. Eur J Immunol 28:1426–1430
De Wit D, Tonon S, Olislagers V, Goriely S, Boutriaux M, Goldman M et al (2003) Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun 21:277–281
Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML (1999) T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today 20:561–267
Wegmann TG, Lin H, Guilbert L, Mosmann TR (1993) Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 14:353–356
Guller S, LaChapelle L (1999) The role of placental Fas ligand in maintaining immune privilege at maternal-fetal interfaces. Semin Reprod Endocrinol 17:39–44
Zarnani AH, Moazzeni SM, Shokri F, Salehnia M, Jeddi-Tehrani M (2007) Kinetics of murine decidual dendritic cells. Reproduction 133:275–283
Shojaeian J, Moazzeni SM, Nikoo S, Bozorgmehr M, Nikougoftar M, Zarnani AH (2007) Immunosuppressive effect of pregnant mouse serum on allostimulatory activity of dendritic cells. J Reprod Immunol 75:23–31
Zarnani AH, Moazzeni SM, Shokri F, Salehnia M, Dokouhaki P, Ghods R, et al (2008) Microenvironment of the feto-maternal interface protects the semiallogenic fetus through its immunomodulatory activity on dendritic cells. Fertil Steril 90:781–788
Niederwieser D, Herold M, Woloszczuk W, Aulitzky W, Meister B, Tilg H et al (1990) Endogenous IFN-gamma during human bone marrow transplantation. Analysis of serum levels of interferon and interferon-dependent secondary messages. Transplantation 50:620–625
Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C (2000) Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood 95:2484–2490
Paiva A, Ferreira T, Freitas A, Couceiro A, Coimbra H, Regateiro FJ (2000) Profile of cytokine production in human cord blood and peripheral blood from healthy donors before and after allogeneic activation: relevance in predicting graft-versus-host disease. Transplant Proc 32:2626–2630
Paiva A, Freitas A, Loureiro A, Couceiro A, Martinho A, Simoes O et al (1998) Functional aspects of cord blood lymphocytes response to polyclonal and allogeneic activation. Bone Marrow Transplant 22(Suppl 1):S31–S34
Encabo A, Solves P, Carbonell-Uberos F, Miñana MD (2007) The functional immaturity of dendritic cells can be relevant to increased tolerance associated with cord blood transplantation. Transfusion 47:272–279
Takahata Y, Nomura A, Takada H, Ohga S, Furuno K, Hikino S et al (2004) CD25+CD4+T cells in human cord blood: an immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol 32:622–629
Chang CC, Satwani P, Oberfield N, Vlad G, Simpson LL, Cairo MS (2005) Increased induction of allogeneic-specific cord blood CD4 + CD25 + regulatory T (Treg) cells: a comparative study of naïve and antigenic-specific cord blood Treg cells. Exp Hematol 33:1508–1520
Acknowledgments
The authors would like to thank the obstetric staff of the Shriati Hospital, for their assistance with cord blood collection and Mrs. Nikougofatar for her invaluable technical assistance in flow cytometry. This work was supported by a grant from the Iranian Blood Transfusion Organization Research Center and Hematology, Oncology and BMT Research Center of Tehran University of Medical Sciences.
Conflict of interest statement
The authors declare that they have no conflict of interest related to the publication of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naderi, N., Pourfathollah, A.A., Alimoghaddam, K. et al. Cord blood dendritic cells prevent the differentiation of naïve T-helper cells towards Th1 irrespective of their subtype. Clin Exp Med 9, 29–36 (2009). https://doi.org/10.1007/s10238-008-0020-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-008-0020-2