Abstract
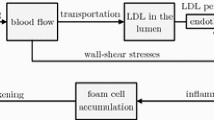
We consider a computational multiscale framework of a bio-chemo-mechanical model for intimal hyperplasia. With respect to existing models, we investigate the interactions between hemodynamics, cellular dynamics and biochemistry on the development of the pathology. Within the arterial wall, we propose a mathematical model consisting of kinetic differential equations for key vascular cell types, collagen and growth factors. The luminal hemodynamics is modeled with the Navier–Stokes equations. Coupling hypothesis among time and space scales are proposed to build a tractable modeling of such a complex multifactorial and multiscale pathology. A one-dimensional numerical test-case is presented for validation by comparing the results of the framework with experiments at short and long timescales. Our model permits to capture many cellular phenomena which have a central role in the physiopathology of intimal hyperplasia. Results are quantitatively and qualitatively consistent with experimental findings at both short and long timescales.










Similar content being viewed by others
Availability of data and material
Python codes used in this study are available at https://gitlab.com/jrme.jansen/pytg/.
Code availability
All simulation has been achieved with in-house Python packages. https://gitlab.com/jrme.jansen/pytg/.
References
Andrews A, Jaron D, Buerk D, Kirby P, Barbee K (2010) Direct, real-time measurement of shear stress-induced nitric oxide produced from endothelial cells in vitro. Nitric Oxide Biol Chem 23(4):335–42. https://doi.org/10.1016/j.niox.2010.08.003
Beamish J, He P, Kottke-Marchant K, Marchant R (2010) Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev 16:467–91. https://doi.org/10.1089/ten.TEB.2009.0630
Bellini C, Ferruzzi J, Roccabianca S, Di Martino E, Humphrey J (2013) A microstructurally motivated model of arterial wall mechanics with mechanobiological implications. Ann Biomed Eng. https://doi.org/10.1007/s10439-013-0928-x
Bennett M, O’Sullivan M (2001) Mechanisms of angioplasty and stent restenosis: implications for design of rational therapy. Pharmacol Ther 91(2):149–166. https://doi.org/10.1016/S0163-7258(01)00153-X
Berk B (2001) Vascular smooth muscle growth: autocrine growth mechanisms. Physiol Rev 81(3):999–1030. https://doi.org/10.1152/physrev.2001.81.3.999 (pMID: 11427690)
Bernhardt J, Tschudi M, Dohi Y, Gut I, Ba Urwyler (1991) Release of nitric oxide from human vascular smooth muscle cells. Biochem Biophys Res Commun 180(2):907–912. https://doi.org/10.1016/S0006-291X(05)81151-9
Binder B, Simpson M (2016) Cell density and cell size dynamics during in vitro tissue growth experiments: implications for mathematical models of collective cell behaviour. Appl Math Model 40(4):3438–3446. https://doi.org/10.1016/j.apm.2015.01.016
Boyle C, Lennon A, Early M, Kelly D, Lally C, Prendergast P (2010) Computational simulation methodologies for mechanobiological modelling: a cell-centred approach to neointima development in stents. Philos Trans A Math Phys Eng Sci 368:2919–2935. https://doi.org/10.1098/rsta.2010.0071
Buchanan C, Verbridge S, Vlachos P, Rylander M (2014) Flow shear stress regulates endothelial barrier function and expression of angiogenic factors in a 3D microfluidic tumor vascular model. Cell Adhes Migr. https://doi.org/10.4161/19336918.2014.970001
Bulelzai M, Dubbeldam J (2012) Long time evolution of atherosclerotic plaques. J Theor Biol 297:1–10. https://doi.org/10.1016/j.jtbi.2011.11.023
Calvez V, Houot J, Meunier N, Raoult A, Rusnakova G (2010) Mathematical and numerical modeling of early atherosclerotic lesions. ESAIM Proc 30:1–14. https://doi.org/10.1051/proc/2010002
Chiu J, Chien S (2011) Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91:327–387. https://doi.org/10.1152/physrev.00047.2009
Clowes A (1993) Chapter 17 intimal hyperplasia and graft failure. Cardiovasc Pathol 2(3, Supplement):179–186. https://doi.org/10.1016/1054-8807(93)90058-A
Clowes A, Reidy M, Clowes M (1983a) Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest 49(3):327–333
Clowes A, Reidy M, Clowes M (1983b) Mechanisms of stenosis after arterial injury. Lab Invest 49(3):208–215
Conklin B, Chen C (2007) Effect of low shear stress on permeability and occludin expression in porcine artery endothelial cells. World J Surg 31:733–743. https://doi.org/10.1007/s00268-006-0735-8
Cornelissen A, Vogt F (2019) The effects of stenting on coronary endothelium from a molecular biological view: time for improvement? J Cell Mol Med 23(1):39–46. https://doi.org/10.1111/jcmm.13936
Cyron C, Aydin R, Humphrey J (2016) A homogenized constrained mixture (and mechanical analog) model for growth and remodeling of soft tissue. Biomech Model Mechanobiol 15(6):1389–1403. https://doi.org/10.1007/s10237-016-0770-9
Davies M (2019) Rutherford’s vascular surgery and endovascular therapy. Elsevier. Chap 5 : Intimal Hyperplasia
de Vries M, Simons K, Jukema J, Braun J, Quax P (2016) Vein graft failure: from pathophysiology to clinical outcomes. Nat Rev Cardiol 13:451–470. https://doi.org/10.1038/nrcardio.2016.76
Donadoni F, Pichardo-Almarza C, Bartlett M, Dardik A, Homer-Vanniasinkam S, Díaz-Zuccarini V (2017) Patient-specific, multi-scale modeling of neointimal hyperplasia in vein grafts. Front Physiol 8:226. https://doi.org/10.3389/fphys.2017.00226
Ducasse E, Eschwege J, Chevalier J, de Ravignan D, Puppinck P, Lartigau E (2003) Hyperplasie intimale artérielle par prolifération de cellules musculaires lisses dans la paroi: données actuelles, traitements expérimentaux et perspectives. J des Maladies Vasculaires 28:130–144. DOI JMV-06-2003-28-3-0398-0499-101019-ART6
Escuer J, Martínez M, McGinty S, Peña E (2019) Mathematical modelling of the restenosis process after stent implantation. J R Soc Interface 16(157):20190313. https://doi.org/10.1098/rsif.2019.0313
Farb A, Kolodgie F, Hwang J, Burke A, Tefera K, Weber D, Wight T, Virmani R (2004) Extracellular matrix changes in stented human coronary arteries. Circulation 110(8):940–947. https://doi.org/10.1161/01.cir.0000139337.56084.30
Feitosa MCP, de Sousa Lima VB, Neto JMM, do Nascimento Marreiro D (2013) Plasma concentration of IL-6 and TNF-alpha and its relationship with zincemia in obese women. Rev Assoc Med Bras 59:429–434. https://doi.org/10.1016/j.ramb.2013.03.003
Feugier P, Rouvière O (2018) Pathologie vasculaire du sportif de haut niveau: endofibrose artérielle. EMC-Angéiologie 1750(19):1–10. https://doi.org/10.1016/S2211-0364(18)85302-X
Fingerle J, Tina AuY, Clowes A, Reidy M (1990) Intimal lesion formation in rat carotid arteries after endothelial denudation in absence of medial injury. Arterioscler Off J Am Assoc Inc 10(6):1082–1087. https://doi.org/10.1161/01.ATV.10.6.1082
Forte A, Della Corte A, De Feo M, Cerasuolo F, Cipollaro M (2010) Role of myofibroblasts in vascular remodelling: focus on restenosis and aneurysm. Cardiovasc Res 88(3):395–405. https://doi.org/10.1093/cvr/cvq224
Fung Y (1993) Mechanical properties and active remodeling of blood vessels. Springer, New York, pp 321–391. https://doi.org/10.1007/978-1-4757-2257-4_8
Garipcan B, Maenz S, Pham T, Settmacher U, Jandt K, Zanow J, Bossert J (2011) Image analysis of endothelial microstructure and endothelial cell dimensions of human arteries—a preliminary study. Adv Eng Mater 13(1–2):B54–B57. https://doi.org/10.1002/adem.201080076
Glagov S, Grande J, Vesselinovitch D, Zarins C (1981) Quantitation of cells and fibers in histologic sections of arterial walls: advantages of contour tracing on a digitizing plate. Springer, New York, pp 57–93. https://doi.org/10.1007/978-1-4612-5967-1_2
Goodman M, Luo X, Hill N (2016) Mathematical model on the feedback between wall shear stress and intimal hyperplasia. Int J Appl Mech 8(7)
Hahn C, Schwartz M (2009) Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10:53–62. https://doi.org/10.1038/nrm2596
Humphrey J (2008) Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem Biophys 50:53–78. https://doi.org/10.1007/s12013-007-9002-3
Humphrey JD (2002) Cardiovascular solid mechanics. Springer, New York. https://doi.org/10.1007/978-0-387-21576-1
Irons L, Humphrey J (2020) Cell signaling model for arterial mechanobiology. PLoS Comput Biol 16(8):1–22. https://doi.org/10.1371/journal.pcbi.1008161
Ivanovic V, Todorovic-Rakovic N, Demajo M, NK Z (2003) Elevated plasma levels of transforming growth factor-b1 (TGF-b1) in patients with advanced breast cancer: association with disease progression. Eur J Cancer 39(4):454–461. https://doi.org/10.1016/S0959-8049(02)00502-6
Jonsson A, Hjalmarsson C, Falk P (2016) Levels of matrix metalloproteinases differ in plasma and serum-aspects regarding analysis of biological markers in cancer. Br J Cancer 67:703–706. https://doi.org/10.1038/bjc.2016.127
Karner G, Perktold K (2000) Effect of endothelial injury and increased blood pressure on albumin accumulation in the arterial wall: a numerical study. J Biomech 33:709–715. https://doi.org/10.1016/S0021-9290(99)00226-2
Kenagy R (2011) Mechanisms of vascular disease. University of Adelaide Press. Chap 7: Biology of Restenosis and Targets for Intervention
Khosravi R, Ramachandra A, Szafron J, Schiavazzi D, Breuer C, Humphrey J (2020) A computational bio-chemo-mechanical model of in vivo tissue-engineered vascular graft development. Integr Biol 12(3):47–63. https://doi.org/10.1093/intbio/zyaa004
Larsson A, Sköldenberg E, Ericson H (2002) Serum and plasma levels of FGF-2 and VEGF in healthy blood donors. Angiogenesis 5:107–110
Lemson M, Tordoir J, Daemen M, Kitslaar P (2000) Intimal hyperplasia in vascular grafts. Eur J Vasc Endovasc Surg 19(4):336–350. https://doi.org/10.1053/ejvs.1999.1040
Levy B, Benessiano J, Henrion D, Caputo L, Heymes C, Duriez M, Poitevin P, Samuel J (1996) Chronic blockade of AT2-subtype receptors prevents the effect of angiotensin II on the rat vascular structure. J Clin Investig 98(2):418–425. https://doi.org/10.1172/JCI118807
Li Y, Haga J, Chien S (2005) Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech 38(10):1949–1971. https://doi.org/10.1016/j.jbiomech.2004.09.030
Li L, Zhang X, Li X, Lv C, Yu H, Xu M, Zhang M, Fu Y, Meng H, Zhou J (2016) TGF-beta inhibits the apoptosis of pulmonary arterial smooth muscle cells and contributes to pulmonary vascular medial thickening via the PI3K/Akt pathway. Mol Med Rep 13(3):2751–2756. https://doi.org/10.3892/mmr.2016.4874
Lindner V, Fingerle J, Reidy M (1993) Mouse model of arterial injury. Circ Res 73(5):792–796. https://doi.org/10.1161/01.res.73.5.792
Liu X, Wang Z, Zhao P, Fan Z, Sun A, Zhan F, Fan Y, Deng X (2014) Nitric oxide transport in normal human thoracic aorta: effects of hemodynamics and nitric oxide scavengers. PLoS ONE 9(11):e112395
Magid R, Murphy T, Galis Z (2003) Expression of matrix metalloproteinase-9 in endothelial cells is differentially regulated by shear stress role of c-Myc. J Biol Chem 278:32994–32999. https://doi.org/10.1074/jbc.M304799200
Malek A, Izumo S (1992) Physiological fluid shear stress causes downregulation of endothelin-1 mRNA in bovine aortic endothelium. Am J Physiol 263(2):C389-96. https://doi.org/10.1152/ajpcell.1992.263.2.C389
Malek A, Gibbons G, Dzau V, Izumo S (1993) Fluid shear stress differentially modulates expression of genes encoding basic fibroblast growth factor and platelet-derived growth factor B chain in vascular endothelium. J Clin Investig 92:2013–2021. https://doi.org/10.1172/JCI116796
Marino M, Pontrelli G, Vairo G, Wriggers P (2017) A chemo-mechano-biological formulation for the effects of biochemical alterations on arterial mechanics: the role of molecular transport and multiscale tissue remodelling. J R Soc Interface 14(136):20170615. https://doi.org/10.1098/rsif.2017.0615
Michel J (2004) Système rénine-angiotensine et remodelage vasculaire. Med Sci 20(4):409–413. https://doi.org/10.1051/medsci/2004204409
Millette E, Rauch B, Defawe O, Kenagy R, Daum G, Clowes A (2005) Platelet-derived growth factor-BB-induced human smooth muscle cell proliferation depends on basic FGF release and FGFR-1 activation. Circ Res 96:172–179. https://doi.org/10.1161/01.RES.0000154595.87608.db
Model LS, Dardik A (2012) Haimovici’s vascular surgery. John Wiley and Sons, Ltd. Chap 10: Neointimal Hyperplasia: Basic Considerations, pp 178–196. https://doi.org/10.1002/9781118481370.ch10
Nagaraja S, Wallqvist A, Reifman J, Mitrophanov A (2014) Computational approach to characterize causative factors and molecular indicators of chronic wound inflammation. J Immunol 192(4):1824–1834. https://doi.org/10.4049/jimmunol.1302481
Newby A (2006) Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res 69:614–624. https://doi.org/10.1016/j.cardiores.2005.08.002
O’Connell M, Murthy S, Phan S, Xu C, Buchanan J, Spilker R, Dalman R, Zarins C, Denk W, Taylor C (2008) The three-dimensional micro- and nanostructure of the aortic medial lamellar unit measured using 3D confocal & electron microscopy imaging. Matrix Biol J Int Soc Matrix Biol 27:171–181. https://doi.org/10.1016/j.matbio.2007.10.008
Ojha M, Leask R, Johnston K, David T, Butany J (2000) Histology and morphology of 59 internal thoracic artery grafts and their distal anastomoses. Ann Thorac Surg 70:1338–1344. https://doi.org/10.1016/S0003-4975(00)01975-5
Olgac U, Kurtcuoglu V, Poulikakos D (2008) Computational modeling of coupled blood-wall mass transport of LDL: effects of local wall shear stress. Am J Physiol Heart Circ Physiol 294(2):H909–H919. https://doi.org/10.1152/ajpheart.01082.2007
Parks WC, Wilson CL, López-Boado YS (2004) Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 4(8):617–629. https://doi.org/10.1038/nri1418
Plata A, Sherwin S, Krams R (2010) Endothelial nitric oxide production and transport in flow chambers: the importance of convection. Ann Biomed Eng 38:2805–2816. https://doi.org/10.1007/s10439-010-0039-x
Qi Y, Jiang J, Jiang X, Wang X (2011) PDGF-BB and TGF-beta1 on cross-talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress. Proc Natl Acad Sci USA 108(5):1908–1913. https://doi.org/10.1073/pnas.1019219108
Qiu J, Zheng Y, Hu J, Liao D, Gregersen H, Deng X, Fan Y, Wang G (2014) Biomechanical regulation of vascular smooth muscle cell functions: from in vitro to in vivo understanding. J R Soc Interface 11(90):20130852. https://doi.org/10.1098/rsif.2013.0852
Raines E (2004) PDGF and cardiovascular disease. Cytokine Growth Factor Rev 15(4):237–254. https://doi.org/10.1016/j.cytogfr.2004.03.004
Raines E, Ross R (1996) Multiple growth factors are associated with lesions of atherosclerosis: specificity or redundancy? BioEssays 18(4):271–282. https://doi.org/10.1002/bies.950180405
Rectenwald E, Moldawer L, Huber S, Seeger M, Ozaki K (2000) Direct evidence for cytokine involvement in neointimal hyperplasia. Circulation 102:1697–1702. https://doi.org/10.1161/01.CIR.102.14.1697
Rhoads D, Eskin S, McIntire L (2000) Fluid flow releases fibroblast growth factor-2 from human aortic smooth muscle cells. Arterioscler Thromb Vasc Biol 20(2):416–421. https://doi.org/10.1161/01.ATV.20.2.416
Robertson A, Sequeira A, Owens R (2009) Cardiovascular mathematics modeling and simulation of the circulatory system. Springer. Chap 6: Rheological models for blood
Rodriguez E, Hoger A, McCulloch A (1994) Stress-dependent finite growth in soft elastic tissues. J Biomech 27(4):455–467. https://doi.org/10.1016/0021-9290(94)90021-3
Rognoni E, Pisco A, Hiratsuka T, Sipilä K, Belmonte J, Mobasseri S, Philippeos C, Dilão R, Watt F (2018) Fibroblast state switching orchestrates dermal maturation and wound healing. Mol Syst Biol 14(8):e8174. https://doi.org/10.15252/msb.20178174
Rossi E, Casali B, Regolisti G, Davoli S, Perazzoli F, Negro A, Sani C, Tumiati B, Nicoli D (1998) Increased plasma levels of platelet-derived growth factor (PDGF-BB + PDGF-AB) in patients with never-treated mild essential hypertension. Am J Hypertens 11(10):1239–1243. https://doi.org/10.1016/s0895-7061(98)00124-1
San Martin A, Hilenski L, Griendling K (2012) Muscle 2-volume set. Elsevier. Chap 96: Molecular Pathways of Smooth Muscle Disease, pp 1279–1287
Schwartz R, Chu A, Edwards W, Srivatsa S, Simari R, Isner J, Holmes D (1996) A proliferation analysis of arterial neointimal hyperplasia: lessons for antiproliferative restenosis therapies. Int J Cardiol 53:71–80. https://doi.org/10.1016/0167-5273(95)02499-9
Shampine L, Thompson S (2009) Numerical solution of delay differential equations. Springer US, Boston, pp 1–27. https://doi.org/10.1007/978-0-387-85595-0_9
Sigg DC (2005) Handbook of cardiac anatomy, physiology, and devices. Humana Press. Chap Cardiac and Vascular Receptors and Signal Transduction, pp 149–159. https://doi.org/10.1007/978-1-59259-835-9_11
Silva T, Jäger W, Neuss-Radu M, Sequeira A (2020) Modeling of the early stage of atherosclerosis with emphasis on the regulation of the endothelial permeability. J Theor Biol 496:110229. https://doi.org/10.1016/j.jtbi.2020.110229
Sporn M, Roberts A (1991) Peptide growth factors and their receptors I. Springer, New York. https://doi.org/10.1007/978-1-4612-3210-0
Starbuck C, Lauffenburger D (1992) Mathematical model for the effects of epidermal growth factor receptor trafficking dynamics on fibroblast proliferation responses. Biotechnol Prog 8(2):132–143. https://doi.org/10.1021/bp00014a007
Taber L (1998) A model for aortic growth based on fluid shear and fiber stresses. J Biomech Eng 120:348–354. https://doi.org/10.1115/1.2798001
Tedgui A, Mallat Z (2006) Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev 86:515–581. https://doi.org/10.1152/physrev.00024.2005
Ultman J, Baskaran H, Saidel G (2016) Biomedical mass transport and chemical reaction: physicochemical principles and mathematical modeling. John Wiley & Sons. Chap 17: Cell Population Dynamics
van Varik B, Rennenberg J, Reutelingsperger C, Kroon A, de Leeuw P, Schurgers L (2012) Mechanisms of arterial remodeling: lessons from genetic diseases. Front Genet 3:290. https://doi.org/10.3389/fgene.2012.00290
Wentzel J, Gijsen F, Stergiopulos N, Serruys P, Slager C, Krams R (2003) Shear stress, vascular remodeling and neointimal formation. J Biomech 36(5):681–688. https://doi.org/10.1016/S0021-9290(02)00446-3
Widmaier E, Raff H, Strang K (2016) Vander’s human physiology. Mc Graw Hill. Chap 1. Homeostasis, pp 1–16
Wilstein Z, Alligood D, McLure V, Miller A (2018) Mathematical model of hypertension-induced arterial remodeling: a chemo-mechanical approach. Math Biosci 303:10–25. https://doi.org/10.1016/j.mbs.2018.05.002
Zhang M, Zhou Y, Chen L, Wang Y, Wang X, Pi Y, Gao C, Li J, Zhang L (2016) An overview of potential molecular mechanisms involved in VSMC phenotypic modulation. Histochem Cell Biol 145:119–130. https://doi.org/10.1007/s00418-015-1386-3
Acknowledgements
We acknowledge fruitful discussions with Dr Lara Chalabreysse and Dr Pauline Baillard about the physiopathology of intimal hyperplasia and endofibrosis.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
X. Escriva and P. Feugier set up the relevant framework for tackling the modeling of the physiological-mechanical pathology. J. Jansen developed the model, carried out simulations of the test-case and wrote the manuscript. F. Godeferd, X. Escriva and P. Feugier participated in the discussion of the results. J. Jansen and F. Godeferd wrote the first draft of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
This article does not present research with ethical considerations.
Consent to participate
All the authors consented to participate in this study.
Consent for publication
All authors proof-read and consent for the publication of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A: Analytical development about diffusion–reaction equation
1.1 Appendix A.1: Dimensional analysis
We use cylindrical coordinates \((r,\theta ,z)\), with r the radius, \(\theta \) the polar angle, and z the axial coordinate. The unsteady diffusion–reaction equation that describes the evolution of the concentration C of a growth factor (GF) x within an idealized arterial wall, which assumes the only radial dependence of C, is
D is the x diffusion coefficient within the arterial wall, and k is the consumption rate of the medium. The idealized arterial wall and domain \(\varOmega _w\) are described in Fig. 1.
The dimensionless unsteady diffusion–reaction equation reads
with the dimensionless variables
\(\widetilde{C}\), L and \(T_{\mathrm {gr}}\) are a characteristic concentration, a characteristic diffusion length and a characteristic growth timescale. The dimensionless consumption is \(k^\dagger =k L^2/D\), and the dimensionless ratio \(L^2/(T_{\mathrm {gr}}D)\) expresses diffusion timescale versus growth timescale.
Upon assuming short growth timescale \(T_{\mathrm {gr}} \sim {1}\) day, thickness of arterial wall \(L={500}\) \(\upmu \)m and NO diffusion coefficient \(D= {8.48 \times 10^{-10}}\) m\(^{2}\) s\(^{-1}\) , the dimensionless number \(L^2/(T_{\mathrm {gr}}D)= {3 \times 10^{-3}}\) remains much lower than one. According to this, we can remove the time-dependent term in Eq. (34)—this was also done in Goodman et al. (2016)—and we get the following steady diffusion reaction
Note that even accounting for a transitional timescale of endothelial cells (ECs) adaptation to hemodynamics, which would be much smaller than \(T_{\mathrm {gr}}\) as \(T_{\mathrm {tr}}\sim 1 \,\mathrm {h}\) (Hahn and Schwartz 2009), the time derivative term in (34) remains negligible (\(L^2/(T_{\mathrm {tr}}D)={8 \times 10^{-2}}\)).
1.2 Appendix A.2: Development of source term expression
The complete problem to be solved is Eq. (37) plus the following Neumann boundary conditions
to model ECs influence on the x GF bioavailability within arterial layers. Equations (38a) and (38b) are, respectively, a flux conservation condition (see Eq. (23)) and a no flux condition at external arterial radius.
Equation (37) is a Bessel equation, whose solution with boundary conditions (38) is
with \(\kappa ^2=k/D\), \(I_{0,1}, \; K_{0,1}\) modified Bessel functions of first and second kind. The vector mass flux comes from Fick’s law \(\varvec{J} = -D \varvec{\nabla } C\), and its amplitude in the radial direction \(\varvec{n}\) comes from (39) as
To define the source term of the generic Eq. (14), we integrate J over the surface of length \(\varDelta z\) at radius r, which amounts to multiplying by \(2 \pi r \varDelta z\) as
m is the production rate of GF as a function of the radial coordinate. To get average production rates of GF x over the layers considered , namely the intima and the media, Eq. (41) is integrated over the layers thicknesses, as in (15). This averaging procedure and the radial variation of \(m^{x}\) are shown in Fig. 11.
\(m^{x}\) from Eq. (41) for \(r\in [R_{\mathrm {l}},R_{\mathrm {EEL}}]\). The averaged values of \(m^{x}\) (from Eq. (15)) within intima and media layers, \(\bar{M}^{x}_{\mathrm {i,m}}\), are represented with dashed lines
Appendix B: Dynamics of growth factors
For reference, we show in Fig. 12 the evolution of all the GFs in both media and intima layers for the test-case presented in Sect. 3, between 0 and 30 days. The initial damage of ECs layer causes a subexpression of NO amount (Lemson et al. 2000) and overexpression of other GFs at short timescale (Ducasse et al. 2003). There are two main types of time evolution for GFs, that of NO and that of other GFs (PDGF, FGF, Ag, TGF, TNF, MMP). From an analysis of biochemical equations, the NO is not involved in inter-GFs coupling mechanisms, so its dynamics is mainly driven by hemodynamics stimuli. This is seen between 6 and 30 days, where there is a net increase in NO amount within intima layer corresponding to the luminal narrowing phase (as WSS increases, so does the production rate). In the second group, a different type of temporal evolution is seen because this group is strongly coupled (seen Table 2). They evolve globally in the same way with an increase in the short time induced by the denudation of the endothelium and a decrease below the equilibrium value \(\delta ^{\mathrm {GFs}}=1.0\) around day 21 which corresponds to the turning point (\(r_{\mathrm {i}} < 0\)) discussed in Sect. 3.2.
Appendix C: Final equilibrium state of the test-case
The final equilibrium state of the denudation test-case developed in Sect. 3 is presented in Table 5. We provide the vector of variables \(\varvec{y}\) of (18) as \(\varvec{y}^\dagger = \varvec{y} / \varvec{y}^{\mathrm {ref}}\) where \(\varvec{y}^\dagger \) is the vector of rescaled or dimensionless variables by its initial–physiological values \(\varvec{y}^{\mathrm {ref}}\).
Rights and permissions
About this article
Cite this article
Jansen, J., Escriva, X., Godeferd, F. et al. Multiscale bio-chemo-mechanical model of intimal hyperplasia. Biomech Model Mechanobiol 21, 709–734 (2022). https://doi.org/10.1007/s10237-022-01558-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-022-01558-5