Abstract
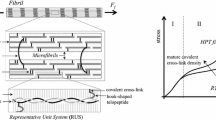
It has been established that the enzyme susceptibility of collagen, the predominant load-bearing protein in vertebrates, is altered by applied tension. However, whether tensile force increases or decreases the susceptibility to enzyme is a matter of contention. It is critical to establish a definitive understanding of the direction and magnitude of the force versus catalysis rate (k C ) relationship if we are to properly interpret connective tissue development, growth, remodeling, repair, and degeneration. In this investigation, we examine collagen/enzyme mechanochemistry at the smallest scale structurally relevant to connective tissue: the native collagen fibril. A single-fibril mechanochemical erosion assay with nN force resolution was developed which permits detection of the loss of a few layers of monomer from the fibril surface. Native type I fibrils (bovine) held at three levels of tension were exposed to Clostridium histolyticum collagenase A. Fibrils held at zero-load failed rapidly and consistently (20 min) while fibrils at 1.8 pN/monomer failed more slowly (35–55 min). Strikingly, fibrils at 23.9 pN/monomer did not exhibit detectable degradation. The extracted force versus k C data were combined with previous single-molecule results to produce a “master curve” which suggests that collagen degradation is governed by an extremely sensitive mechanochemical switch.
Similar content being viewed by others
References
Adhikari AS, Chai J, Dunn AR (2011) Mechanical load induces a 100-fold increase in the rate of collagen proteolysis by MMP-1. J Am Chem Soc. doi:10.1021/ja109972p
Arokoski JP, Jurvelin JS, Vaatainen U, Helminen HJ (2000) Normal and pathological adaptations of articular cartilage to joint loading. Scand J Med Sci Sports 10(4): 186–198
Baragi VM, Qiu L, Gunja-Smith Z, Woessner JF Jr., Lesch CA, Guglietta A (1997) Role of metalloproteinases in the development and healing of acetic acid-induced gastric ulcer in rats. Scand J Gastroenterol 32(5): 419–426
Bass EC, Wistrom EV, Diederich CJ, Nau WH, Pellegrino R, Ruberti J, Lotz JC (2004) Heat-induced changes in porcine annulus fibrosus biomechanics. J biomech 37(2): 233–240
Beare AH, O’Kane S, Krane SM, Ferguson MW (2003) Severely impaired wound healing in the collagenase-resistant mouse. J Invest Dermatol 120(1): 153–163. doi:10.1046/j.1523-1747.2003.12019.x
Bell E, Ivarsson B, Merrill C (1979) Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA 76(3): 1274–1278
Bhole AP, Flynn BP, Liles M, Saeidi N, Dimarzio CA, Ruberti JW (2009) Mechanical strain enhances survivability of collagen micronetworks in the presence of collagenase: implications for load-bearing matrix growth and stability. Philos Trans A Math Phys Eng Sci 367(1902): 3339–3362
Blain EJ, Gilbert SJ, Wardale RJ, Capper SJ, Mason DJ, Duance VC (2001) Up-regulation of matrix metalloproteinase expression and activation following cyclical compressive loading of articular cartilage in vitro. Arch Biochem Biophys 396(1): 49–55
Camp RJ, Liles M, Beale J, Saeidi N, Flynn BP, Moore E, Murthy SK, Ruberti JW (2011) Molecular mechanochemistry: low force switch slows enzymatic cleavage of human type I collagen monomer. J Am Chem Soc. doi:10.1021/ja110098b
Canty EG, Lu Y, Meadows RS, Shaw MK, Holmes DF, Kadler KE (2004) Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol 165(4): 553–563. doi:10.1083/jcb.200312071
Cawston T, Billington C, Cleaver C, Elliott S, Hui W, Koshy P, Shingleton B, Rowan A (1999) The regulation of MMPs and TIMPs in cartilage turnover. Ann NY Acad Sci 878: 120–129
Chang S-W, Flynn BP, Ruberti JW, Buehler MJ (2012) Molecular mechanism of force induced stabilization of collagen against enzymatic breakdown. Biomaterials 33(15): 3852–3859
Chen SS, Wright NT, Humphrey JD (1997) Heat-induced changes in the mechanics of a collagenous tissue: isothermal free shrinkage. J Biomech Eng 119(4): 372–378
Cowin SC (2004) Tissue growth and remodeling. Annu Rev Biomed Eng 6: 77–107
Eppell SJ, Smith BN, Kahn H, Ballarini R (2006) Nano measurements with micro-devices: mechanical properties of hydrated collagen fibrils. J R Soc Interface 3(6): 117–121. doi:10.1098/rsif.2005.0100
Flynn BP, Bhole AP, Saeidi N, Liles M, Dimarzio CA, Ruberti JW (2010) Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8). PLoS One 5(8). doi:10.1371/journal.pone.0012337
Grams F, Reinemer P, Powers JC, Kleine T, Pieper M, Tschesche H, Huber R, Bode W (1995) X-ray structures of human neutrophil collagenase complexed with peptide hydroxamate and peptide thiol inhibitors. Implications for substrate binding and rational drug design. Eur J Biochem 228(3): 830–841
Grytz R, Meschke G, Jonas JB (2011) The collagen fibril architecture in the lamina cribrosa and peripapillary sclera predicted by a computational remodeling approach. Biomech Model Mechanobiol 10(3): 371–382. doi:10.1007/s10237-010-0240-8
Harrington DJ (1996) Bacterial collagenases and collagen-degrading enzymes and their potential role in human disease. Infect Immun 64(6): 1885–1891
Hormann H (1982) Fibronectin–mediator between cells and connective tissue. Klin Wochenschr 60(20): 1265–1277
Huang C, Yannas IV (1977) Mechanochemical studies of enzymatic degradation of insoluble collagen fibers. J Biomed Mater Res Symp 11(1):137–154
Huang Y, Meek KM (1999) Swelling studies on the cornea and sclera: the effects of pH and ionic strength. Biophys J 77(3): 1655–1665. doi:10.1016/S0006-3495(99)77013-X
Hulboy DL, Rudolph LA, Matrisian LM (1997) Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod 3(1): 27–45
Hulmes DJ, Miller A, Parry DA, Piez KA, Woodhead-Galloway J (1973) Analysis of the primary structure of collagen for the origins of molecular packing. J Mol Biol 79(1): 137–148
Humphrey JD (2001) Stress, strain, and mechanotransduction in cells. J Biomech Eng 123(6): 638–641
Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, Lopez-Otin C, Krane SM (2004) Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci USA 101(49): 17192–17197. doi:10.1073/pnas.0407788101
Kadler K (2004) Matrix loading: assembly of extracellular matrix collagen fibrils during embryogenesis. Birth Defects Res C Embryo Today 72(1): 1–11
Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF III, Evans CH (1996) Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976) 21(3):271–277
Kilian O, Pfeil U, Wenisch S, Heiss C, Kraus R, Schnettler R (2007) Enhanced alpha 1(I) mRNA expression in frozen shoulder and dupuytren tissue. Eur J Med Res 12(12): 585–590
Koskinen SO, Heinemeier KM, Olesen JL, Langberg H, Kjaer M (2004) Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon-related connective tissue. J Appl Physiol 96(3): 861–864
Leikina E, Mertts MV, Kuznetsova N, Leikin S (2002) Type I collagen is thermally unstable at body temperature. Proc Natl Acad Sci USA 99(3): 1314–1318. doi:10.1073/pnas.032307099
Lyubimov AV, Vasiliev JM (1982) Distribution of fibronectin-containing structures on the surface of lamelloplasm and endoplasm of fibroblasts; hypothesis of receptor-mediated assembly of fibronectin structures. Cell Biol Int Rep 6(2): 105–112
Mallya SK, Mookhtiar KA, Van Wart HE (1992) Kinetics of hydrolysis of type I, II, and III collagens by the class I and II Clostridium histolyticum collagenases. J Protein Chem 11(1): 99–107
McCawley LJ, Matrisian LM (2000) Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today 6(4): 149–156. doi:S1357-4310(00)01686-5
Meek KM, Leonard DW (1993) Ultrastructure of the corneal stroma: a comparative study. Biophys J 64: 273–280
Michna H, Hartmann G (1989) Adaptation of tendon collagen to exercise. Int Orthop 13(3): 161–165
Miles CA, Ghelashvili M (1999) Polymer-in-a-box mechanism for the thermal stabilization of collagen molecules in fibers. Biophys J 76(6): 3243–3252. doi:10.1016/S0006-3495(99)77476-X
Minond D, Lauer-Fields JL, Nagase H, Fields GB (2004) Matrix metalloproteinase triple-helical peptidase activities are differentially regulated by substrate stability. Biochemistry 43(36): 11474–11481. doi:10.1021/bi048938i
Nabeshima Y, Grood ES, Sakurai A, Herman JH (1996) Uniaxial tension inhibits tendon collagen degradation by collagenase in vitro. J Orthop Res 14: 123–130
Nemetschek T, Jonak R, Nemetschek-Gansler H, Riedl H, Rosenbaum G (1978) [On the determination of changes in the large periodic structure of collagen (author’s transl)]. Z Naturforsch C 33(11-12): 928–936
Nerenberg PS, Salsas-Escat R, Stultz CM (2008) Do collagenases unwind triple-helical collagen before peptide bond hydrolysis? Reinterpreting experimental observations with mathematical models. Proteins 70(4): 1154–1161. doi:10.1002/prot.21687
Okada T, Hayashi T, Ikada Y (1992) Degradation of collagen suture in vitro and in vivo. Biomaterials 13: 448–454
Perumal S, Antipova O, Orgel JP (2008) Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis. Proc Natl Acad Sci USA 105(8): 2824–2829. doi:10.1073/pnas.0710588105
Prajapati RT, Chavally-Mis B, Herbage D, Eastwood M, Brown RA (2000) Mechanical loading regulates protease production by fibroblasts in three-dimensional collagen substrates. Wound Repair Regen 8(3): 226–237
Ruberti JW, Hallab NJ (2005) Strain-controlled enzymatic cleavage of collagen in loaded matrix. Biochem Biophys Res Commun 336(2): 483–489. doi:10.1016/j.bbrc.2005.08.128
Sander EA, Barocas VH, Tranquillo RT (2011) Initial fiber alignment pattern alters extracellular matrix synthesis in fibroblast-populated fibrin gel cruciforms and correlates with predicted tension. Ann Biomed Eng 39(2): 714–729. doi:10.1007/s10439-010-0192-2
Stultz CM (2002) Localized unfolding of collagen explains collagenase cleavage near imino-poor sites. J Mol Biol 319(5): 997–1003. doi:10.1016/S0022-2836(02)00421-7
Tzafriri AR, Bercovier M, Parnas H (2002) Reaction diffusion model of the enzymatic erosion of insoluble fibrillar matrices. Biophys J 83: 776–793
Van der Rijt JA, Van der Werf KO, Bennink ML, Dijkstra PJ, Feijen J (2006) Micromechanical testing of individual collagen fibrils. Macromol Biosci 6(9): 697–702. doi:10.1002/mabi.200600063
Welgus HG, Jeffrey JJ, Stricklin GP, Roswit WT, Eisen AZ (1980) Characteristics of the action of human skin fibroblast collagenase on fibrillar collagen. J Biolo Chem 255: 6808–6813
Wyatt KE, Bourne JW, Torzilli PA (2009) Deformation-dependent enzyme mechanokinetic cleavage of type I collagen. J Biomech Eng 131(5): 051004
Zareian R, Church KP, Saeidi N, Flynn BP, Beale JW, Ruberti JW (2010) Probing collagen/enzyme mechanochemistry in native tissue with dynamic, enzyme-induced creep. Langmuir 26(12): 9917–9926. doi:10.1021/la100384e
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
The Below is the Electronic Supplementary Material.
Rights and permissions
About this article
Cite this article
Flynn, B.P., Tilburey, G.E. & Ruberti, J.W. Highly sensitive single-fibril erosion assay demonstrates mechanochemical switch in native collagen fibrils. Biomech Model Mechanobiol 12, 291–300 (2013). https://doi.org/10.1007/s10237-012-0399-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-012-0399-2