Abstract
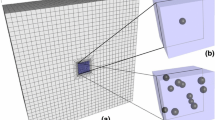
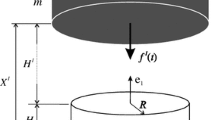
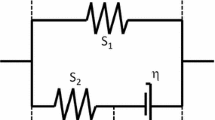
Experimental findings indicate that in-situ chondrocytes die readily following impact loading, but remain essentially unaffected at low (non-impact) strain rates. This study was aimed at identifying possible causes for cell death in impact loading by quantifying chondrocyte mechanics when cartilage was subjected to a 5% nominal tissue strain at different strain rates. Multi-scale modelling techniques were used to simulate cartilage tissue and the corresponding chondrocytes residing in the tissue. Chondrocytes were modelled by accounting for the cell membrane, pericellular matrix and pericellular capsule. The results suggest that cell deformations, cell fluid pressures and fluid flow velocity through cells are highest at the highest (impact) strain rate, but they do not reach damaging levels. Tangential strain rates of the cell membrane were highest at the highest strain rate and were observed primarily in superficial tissue cells. Since cell death following impact loading occurs primarily in superficial zone cells, we speculate that cell death in impact loading is caused by the high tangential strain rates in the membrane of superficial zone cells causing membrane rupture and loss of cell content and integrity.
Similar content being viewed by others
References
Abusara Z, Seerattan R, Leumann A, Thompson R, Herzog W (2011) A novel method for determining articular cartilage chondrocyte mechanics in vivo. J Biomech 44(5): 930–934
Alexopoulos LG, Setton LA, Guilak F (2005) The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater 1(3): 317–325
Alexopoulos LG, Williams GM, Upton ML, Setton LA, Guilak F (2005) Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J Biomech 38(3): 509–517
Ateshian GA, Costa KD, Hung CT (2007) A theoretical analysis of water transport through chondrocytes. Biomech Model Mechanobiol 6(1–2): 91–101
Bevensee MO, Schwiening CJ, Boron WF (1995) Use of bcecf and propidium iodide to assess membrane integrity of acutely isolated ca1 neurons from rat hippocampus. J Neurosci Methods 58(1–2): 61–75
Blanco FJ, Guitian R, Vázquez-Martul E, de Toro FJ, Galdo F (1998) Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthr Rheum 41(2): 284–289
Brandt K, Mankin H, Shulman L (1986) Workshop on etiopathogenesis of osteoarthritis. J Rheumatol 13: 1126–1160
Chahine NO, Ateshian GA, Hung CT (2007) The effect of finite compressive strain on chondrocyte viability in statically loaded bovine articular cartilage. Biomech Model Mechanobiol 6(1–2): 103–111
Duda GN, Eilers M, Loh L, Hoffman JE, Kääb M, Schaser K (2001) Chondrocyte death precedes structural damage in blunt impact trauma. Clin Orthop Relat Res 393: 302–309
Escolar G, Leistikow E, White JG (1989) The fate of the open canalicular system in surface and suspension-activated platelets. Blood 74(6): 1983–1988
Evans EA, Skalak R (1979) Mechanics and thermodynamics of biomembranes: part 1. CRC Crit Rev Bioeng 3(3): 181–330
Ewers BJ, Dvoracek-Driksna D, Orth MW, Haut RC (2001) The extent of matrix damage and chondrocyte death in mechanically traumatized articular cartilage explants depends on rate of loading. J Orthop Res Off Publ Orthop Res Soc 19(5): 779–784
Federico S, Grillo A, Herzog W (2004) A transversely isotropic composite with a statistical distribution of spheroidal inclusions: a geometrical approach to overall properties. J Mech Phys Solids 52(10): 2309–2327
Federico S, Grillo A, Larosa G, Giaquinta G, Herzog W (2005) A transversely isotropic, transversely homogeneous microstructural-statistical model of articular cartilage. Jv Biomech 38(10): 2008–2018
Geddes DM, Cargill RS, LaPlaca MC (2003) Mechanical stretch to neurons results in a strain rate and magnitude-dependent increase in plasma membrane permeability. J Neurotrauma 20(10): 1039–1049
Grillo A, Wittum G, Giaquinta G, Micunovic MV (2009) A multiscale analysis of growth and diffusion dynamics in biological materials. Int J Eng Sci 47(2): 261–283
Guilak F, Mow VC (1992) Determination of the mechanical response of the chondrocyte in situ using finite element modeling and confocal microscopy. ASME Adv Bioeng BED 20: 21–23
Guilak F, Mow VC (2000) The mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions in articular cartilage. J Biomech 33(12): 1663–1673
Guilak F, Ratcliffe A, Mow VC (1995) Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J Orthop Res Off Publ Orthop Res Soc 13(3): 410–421
Han SK, Federico S, Grillo A, Giaquinta G, Herzog W (2007) The mechanical behaviour of chondrocytes predicted with a micro-structural model of articular cartilage. Biomech Model Mechanobiol 6(3): 139–150
Han SK, Federico S, Herzog W (2011) A depth-dependent model of the pericellular microenvironment of chondrocytes in articular cartilage. Comput Methods Biomech Biomed Eng 14(7): 657–664
Hassanizadeh SM (1986) Derivation of basic equations of mass transport in porous media, part 1. Macroscopic balance laws. Adv Water Resour 9(4): 196–206
Hedlund H, Mengarelli-Widholm S, Reinholt FP, Svensson O (1993) Stereologic studies on collagen in bovine articular cartilage. APMIS 101(1–6): 133–140
Holmes MH, Mow VC (1990) The nonlinear characteristics of soft gels and hydrated connective tissues in ultrafiltration. J Biomech 23(11): 1145–1156
Hynes RO (1992) Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69(1): 11–25
Isaac DI, Meyer EG, Haut RC (2008) Chondrocyte damage and contact pressures following impact on the rabbit tibiofemoral joint. J Biomech Eng 130(4): 041,018–041,018
Islam N, Haqqi TM, Jepsen KJ, Kraay M, Welter JF, Goldberg VM, Malemud CJ (2002) Hydrostatic pressure induces apoptosis in human chondrocytes from osteoarthritic cartilage through up-regulation of tumor necrosis factor-alpha, inducible nitric oxide synthase, p53, c-myc, and bax-alpha, and suppression of bcl-2. J Cell Biochem 87(3): 266–278
Jay AW, Canham PB (1977) Viscoelastic properties of the human red blood cell membrane. ii. area and volume of individual red cells entering a micropipette. Biophys J 17(2): 169–178
Kurz B, Jin M, Patwari P, Cheng DM, Lark MW, Grodzinsky AJ (2001) Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res 19(6): 1140–1146
Li L, Herzog W, Korhonen R, Jurvelin J (2005) The role of viscoelasticity of collagen fibers in articular cartilage: axial tension versus compression. Med Eng Phys 27(1): 51–57
Mankin HJ (1963) Localization of tritiated thymidine in articular cartilage of rabbits: Iii. Mature articular cartilage. J Bone Jt Surg Am 45(3): 529–540
Maroudas A, Bullough P (1968) Permeability of articular cartilage. Nature 219(5160): 1260–1261
McNeil PL, Steinhardt RA (2003) Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol 19: 697–731
Milentijevic D, Torzilli PA (2005) Influence of stress rate on water loss, matrix deformation and chondrocyte viability in impacted articular cartilage. J Biomech 38(3): 493–502
Milentijevic D, Helfet DL, Torzilli PA (2003) Influence of stress magnitude on water loss and chondrocyte viability in impacted articular cartilage. J Biomech Eng 125(5): 594–601
Mow VC, Guilak F (1993) Deformation of chondrocytes within the extracellular matrix of articular cartilage. Birkhäuser, Boston, pp 128–145
Mow VC, Kuei SC, Lai WM, Armstrong CG (1980) Biphasic creep and stress relaxation of articular cartilage in compression: theory and experiments. J Biomech Eng 102(1): 73–84
Nakamura S, Arai Y, Takahashi KA, Terauchi R, Ohashi S, Mazda O, Imanishi J, Inoue A, Tonomura H, Kubo T (2006) Hydrostatic pressure induces apoptosis of chondrocytes cultured in alginate beads. J Orthop Res Off Publ Orthop Res Soc 24(4): 733–739
Nguyen BV, Wang Q, Kuiper NJ, El Haj AJ, Thomas CR, Zhang Z (2009) Strain-dependent viscoelastic behaviour and rupture force of single chondrocytes and chondrons under compression. Biotechnol Lett 31(6): 803–809
Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW (1993) Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci USA 90(24): 11,663–11,667
Poole CA, Flint MH, Beaumont BW (1987) Chondrons in cartilage: ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. J Orthop Res Off Publ Orthop Res Soc 5(4): 509–522
Quinn TM, Allen RG, Schalet BJ, Perumbuli P, Hunziker EB (2001) Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. J Orthop Res Off Publ Orthop Res Soc 19(2): 242–249
Raucher D, Sheetz MP (1999) Characteristics of a membrane reservoir buffering membrane tension. Biophys J 77(4): 1992–2002
Repo RU, Finlay JB (1977) Survival of articular cartilage after controlled impact. J Bone Jt Surg A 59(8): 1068–1076
Schinagl RM, Gurskis D, Chen AC, Sah RL (1997) Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res Off Publ Orthop Res Soc 15(4): 499– 506
Sheetz MP, Dai J (1996) Modulation of membrane dynamics and cell motility by membrane tension. Trends Cell Biol 6(3): 85–89
Shi R, Whitebone J (2006) Conduction deficits and membrane disruption of spinal cord axons as a function of magnitude and rate of strain. J Neurophysiol 95(6): 3384–3390
Shieh AC, Athanasiou KA (2006) Biomechanics of single zonal chondrocytes. J Biomech 39(9): 1595–1602
Simon WH, Richardson S, Herman W, Parsons JR, Lane J (1976) Long-term effects of chondrocyte death on rabbit articular cartilage in vivo. J Bone Jt Surg Am 58(4): 517–526
Stroetz RW, Vlahakis NE, Walters BJ, Schroeder MA, Hubmayr RD (2001) Validation of a new live cell strain system: characterization of plasma membrane stress failure. J Appl Physiol 90(6): 2361–2370
Trickey WR, Lee GM, Guilak F (2000) Viscoelastic properties of chondrocytes from normal and osteoarthritic human cartilage. J Orthop Res Off Publ Orthop Res Soc 18(6): 891–898
Wang CCB, Chahine NO, Hung CT, Ateshian GA (2003) Optical determination of anisotropic material properties of bovine articular cartilage in compression. J Biomech 36(3): 339–353
Ward MD, Hammer DA (1993) A theoretical analysis for the effect of focal contact formation on cell-substrate attachment strength. Biophys J 64(3): 936–959
Waugh RE (1983) Effects of abnormal cytoskeletal structure on erythrocyte membrane mechanical properties. Cell Motil 3(5–6): 609–622
Wu JZ, Herzog W (2000) Finite element simulation of location- and time-dependent mechanical behavior of chondrocytes in unconfined compression tests. Ann Biomed Eng 28(3): 318–330
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moo, E.K., Herzog, W., Han, S.K. et al. Mechanical behaviour of in-situ chondrocytes subjected to different loading rates: a finite element study. Biomech Model Mechanobiol 11, 983–993 (2012). https://doi.org/10.1007/s10237-011-0367-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-011-0367-2