Abstract
We studied the physicochemical and biological characteristics of the Dombrovska pit lake in Ukraine. The lake formed in an abandoned opencast potassium salt mine and is one of the most saline inland water bodies in the world. It is 85 m deep (November 2015) and an annual inflow of about 2 Mm3 of water. The lake has two distinct layers. The mesohaline surface (0–5 m) layer is well oxygenated and slightly alkaline (pH = 7.5–8.8). Its mineralization, expressed as dry mass, was 50–134 g dm− 3, and its electrical conductivity (EC) was 58–134 mS cm− 1. The underlying layer consists of hypersaline water with low amounts of dissolved oxygen, a neutral pH (6.7–7.4), high mineralization (179–420 g dm− 3), high EC (169–215 mS cm− 1), and higher concentrations of major anions and cations (except Ca2+) and nutrients than the overlying water. The vertical relationship between major ions and metals and the future salinity of the lake are discussed. In terms of zooplankton, in July we found living specimens of the rotifer Brachionus plicatilis and the ciliates Paradileptus elephantinus and Tindinnidium sp. as well as dead rotifers, cladocerans, and copepods (in total, 19 species), but only live B. plicatilis and 9 dead species in November. In the littoral part of the pit lake, we found the diatoms Nitzschia pusilla and some Halamphora species (H. borealis, H. tenerrima, H. acutiuscula), which favour highly saline waters.
Zusammenfassung
Wie untersuchten die physikochemischen und biologischen Charakteristika des Dombrovska Tagebausees in der Ukraine. Der See entstand im Tagebau einer aufgelassenen Kalisalzmine und ist einer der höchstsalinaren Inlandwasserkörper der Welt. Er ist 85 m tief (November 2015) und hat einen Jahreszufluß von ca. 2 Mm3 Wasser. Der See ist deutlich geschichtet: Die mesohaline Oberflächenschicht (0-5 m) ist sauerstoffreich und leicht alkalisch (pH=7.5-8.8). Die Trockenmasse gelöster Stoffe beträgt 50-134 g L-1 und die elektrische Leitfähigkeit (EC) war 58-134 mS cm-1. Die untere Schicht hypersalinen Wassers enthält bei neutralem pH (6.7-7.4) und hoher EC (169-215 mS cm-1) wenig gelösten Sauerstoff, eine hohe Trockenmasse gelöster Stoffe (179-420 g L-1) und höhere Konzentrationen von wichtigen Anionen und Kationen (außer Ca2+) sowie wichtigen Nährstoffen als das darüberliegende Wasser. Die vertikale Verteilung der Anionen und Kationen und die zukünftige Salinität des Sees werden angesprochen. Zooplankton im Juli umfaßte lebende Specimen der Rotofere Brachionus plicatilis und der Ciliaten Paradileptus elephantinus und Tindinnidium sp., dazu tote Rotifera, Cladocera und Copepoden (insgesamt 19 Species). Im November fanden wir nur lebende B. plicatilis und 9 tote Arten. Im litoralen Bereich des Sees fanden wir die Diatomeen Nitzschia pusilla und einige Halamphora Arten (H. borealis, H. tenerrima, H. acutiuscula), welche hochsalinare Wässer bevorzugen.
Resumen
Hemos estudiado las características fisicoquímicas y biológicas del lago del hoyo de mina Dombrovska en Ucrania. El lago formado en una mina de sal de potasio abandonada es uno de los mayores cuerpos salinos tierras adentro en el mundo. Tiene 85 m de profundidad (Noviembre 2015) y un flujo entrante anual de 2 Mm3 de agua. El lago tiene dos capas diferenciadas. La capa superficial mesosalina (0-5 m) está bien oxigenada y es ligeramente alcalina (pH=7,5-8,8). Su mineralización, expresada en masa seca, era 50-134 g dm-3 y su conductividad eléctrica (EC) era 58-134 mS cm-1. La capa profunda consistente en agua hipersalina con pequeñas cantidades de oxígeno disuelto, presenta pH casi neutro (6,7-7,4), alta mineralización (179-420 g dm-3), alta EC (169-215 mS cm-1) y mayores concentraciones de los principales aniones y cationes (excepto Ca2+) y nutrientes que la capa superficial. Se discute la relación vertical entre los principales iones y metales y la salinidad futura del lago. En términos de zooplancton, en Julio encontramos especímenes vivos del rotífero Brachionus plicatilis y de los ciliados Paradileptus elephantinus y Tindinnidium sp. así como especímenes muertos de rotíferos, cladóceros y copépodos (en total, 19 especies) pero solo B. plicatilis vivo y 9 especies muertas en Noviembre. En la parte litoral del lago, encontramos las diatomeas Nitzschia pusilla y algunas species de Halamphora (H. borealis, H. tenerrima, H. acutiuscula) que son favorecidos por aguas altamente salinas.
Similar content being viewed by others
Introduction
Potash resources come from marine deposits and usually occur deep in the earth. They are typically exploited by conventional shaft mining, dissolution mining, or evaporation from brines. In contrast, the potash ore that occurs near the city of Kalush in the Ukraine (Ivano Frankiv region, Carpathian Foredeep Basin), is located only about 20 m below the surface and is suited to opencast mining (Gaydin 2011). The ore is rich in kainite (KMg (Cl/SO4)·3H2O), halite (NaCl), and langbeinite (K2Mg2(SO4)3).
The Dombrovska open pit mine in Kalush operated from 1967 to 2005. More than 14.7 million tons of potassium salts with an admixture of NaCl were mined up to the 1990s. Mine closure occurred between 2005 and 2008, and included a groundwater collection ditch with a length of 5.3 km and a depth of 25 m dug around the opencast mine. Groundwater was pumped from the ditch to the nearby Sivka River. In 2008, due to bankruptcy of the enterprise, the opencast mine was abandoned and it filled with highly mineralised quaternary water, rain water, and groundwater seepage (Gajdin et al. 2014; Gaydin 2011). Because potassium and sodium salts are highly soluble (360 g kg− 1 of water at 25 °C for both KCl and NaCl), they are naturally concentrated in the pit lake water, creating a unique water chemistry and specific life conditions for biota.
According to Dolin et al. (2010), the Dombrovska pit lake was expected to be saline throughout the whole water column, with water mineralization of 80–115 g dm− 3 in the surface layer and ca. 400 g dm− 3 at a depth of 10–15 m. In contrast, Gajdin et al. (2014) and Gaydin and Diakiv (2010) suggested that, based on physical modeling and theoretical calculations, the filled pit lake would have a freshwater surface layer (up to a depth of 18 m) and saline water only in the deeper layers. In this study, we investigated the chemical composition of the water and the biological life (zooplankton, diatoms). Based on the available literature, we hypothesised that the water chemistry would change as suggested by Gajdin et al. (2014) and Gaydin (2011) with the upper layer of the pit lake becoming less saline.
Materials and Methods
Study Area
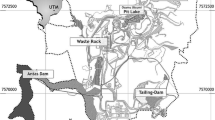
The geological profile of the potassium salt deposit includes three basic substances: potash, salt-bearing breccias of weathered clays, and Quaternary formations. The salts include very soluble halite, kainite, and sylvite, and less soluble langbeinite, kieserite, polyhalite and anhydrite. The salt-bearing breccia consists of clays and sandstones (15–17%) bounded by halite, and contain up to 45% salt. The Quaternary sediments include 2–18 m thick water-bearing gravel layers covered by loams with a thickness of 2.5–6 m. In the southern part of the pit, the slopes consist of salt-bearing breccias and, in the northern part, potash (Fig. 1a, b; Gajdin et al. 2014).
The Dombrovska pit lake is located near the town of Kalush, Ukraine (49°01′34.18″N, 24°19′24.74″E). In November 2015, the lake was 1770 m long, 260–450 m wide, and 85 m deep (Fig. 2). Presently an annual inflow is about 2 million m3 of water every year, causing an annual water level increase of about 4 m (Dolin et al. 2010). The pit lake has already accumulated over 20 million m3 of brine. The slopes of the pit lake include the salt-bearing layer and the clay cap. The salinity is increased by occasional landslides of the clay and Quaternary gravels that cover the shore (Gajdin et al. 2014). The pit lake bottom is covered by a 20 m thick layer of clay.
Sample Collection and Methods
Water samples were collected in 2015 from one station situated at the deepest part of the pit lake. In July, we sampled at depths of 0, 5, 10, 15, 20, 30, 40, 50, 60, 70, and 80 m and in November, at depths of 0, 2.5, 5, 7.5, 10, 15, 20, 30, 40, 50, 60, 70, 80, and 85 m. We determined the following parameters: pH, electrical conductivity (EC), water mineralization, dissolved oxygen (DO), oxygen saturation, and concentrations of anions (Cl−, SO42−, HCO3−, NO3−), cations (Na+, K+, Ca2+, Mg2+, NH4+), and metals (Cd, Pb, Zn, Mn and Fe).
The EC and pH were analysed in situ. The concentrations of inorganic ions (Cl−, SO42−, HCO3−, NO3−, Na+, K+, Ca2+, Mg2+) were analysed using ion chromatography (DIONEX ICS 1000 and IC DX 320). Due to the high EC levels, this analysis required considerable dilution (100 and 500×) of the water samples. Therefore, the data for those anions that were present in lesser amounts at certain depths were below the detection limit. Water mineralization was expressed by the amount of dry residue. Light conditions were determined in July using a Secchi disk. The Winkler method was used to determine DO. For metal analysis, samples were acidified to pH < 2 with ultrapure HNO3. The concentrations of Zn, Cd, Pb, Fe, and Mn were measured using a Varian Spectra AA-20 atomic absorption spectrophotometer with atomization in an air-acetylene flame. Chlorophylls were extracted from membrane filters after filtering a known volume of lake water and analysed in 90% acetone at 630, 663, 664, and 647 nm using a spectrophotometer U-1900 with a slit width of 4 nm and a path length (cuvette) of 10 mm. Chlorophylls a and b were calculated using the formulas described in Mitchel and Kiefer (1984) for mixed phytoplankton and chlorophyll c was calculated using the formula for cryptomonads and dinoflagellates: chlorophyll c = 27.09 E630 − 3.63E663, where E630 and E663 are the solution extinction at 630 or 663 nm, respectively.
Because we detected a strong hydrocarbon odour in the deeper water layers, we analysed hydrocarbons in the water samples from 85 to 50 m deep. The analysis was performed by gas chromatography CLARUS 500 with an FID detector according to norm PN-EN ISO 9377-2:2003. For the extraction of oil from water samples, the solvent n-hexane in HPLC grade was used.
Water for biological analysis was taken using a 5 L bathometer from the same depth as the water for physicochemical analyses. We filtered the 15 L water samples through a #50 µm planktonic net. In the first sampling term (July), samples were preserved with 4% formalin. During the second sampling term (November), planktonic samples were not preserved until identification of living organisms was complete. This procedure was due to the difficulty of distinguishing between as-sampled living and dead animals in a preserved sample. Taxonomic identification of ciliates (Ciliata) was carried out in the living material in a water drop with cover glass according to Foissner and Berger (1996) and Foissner et al. (1999). Identification of rotifers (Rotifera), cladocerans, and copepods was carried out using several identification keys (Bielańska-Grajner et al. 2014; Dussart 1969; Flössner 1972; Koste 1978).
Diatoms were determined in the benthic sample of surface sediment covered by algae collected ca. 0.2 m from the shore. The diatoms were identified with a Nikon 80i light microscope on permanent slides, where diatom valves were mounted using the synthetic resin Naphrax®. Diatom identification was based mainly on Krammer and Lange-Bertalot (1986, 1988, 1991, 2004).
Pearson linear correlations were used to estimate the relationship between environmental parameters. The Wilcoxon test was used to determine differences in the water column’s physicochemical parameters between July and November.
Results
Physicochemical Water Parameters
Light conditions were poor in the Dombrovska pit lake in July, with a Secchi depth of only 0.8 m, indicating a euphotic zone of about 2 m. The water temperature varied with depth (Fig. 3). In July, the temperature was high (22–23 °C) at the near-surface water and at ≈ 10 m depth, while the lowest observed temperature in July (14.9 °C) was at a depth of 5 m. Temperature showed little variability (17.0–20.3 °C) below a depth of 10 m. In November, the lowest temperature was at the surface (7.4 °C) while the highest temperature (22 °C) was observed at a depth of 10 m, unchanged from the July reading. Between the depths of 15 and 85 m, water temperature was almost constant (17.1–17.5 °C).
Water pH ranged from 6.7 to 8.0 (Fig. 3). Surface water layers (0–5 m) were slightly alkaline (pH > 7.5), but from depths of 15–85 m, the pH was in the neutral range (6.8–7.4). We observed a gradual increase in pH from 10 m and deeper in July and small changes in November. The pH values were significantly higher in July than in November (Table 1). The DO in the pit lake water ranged from 0.2 to 10.9 mg dm− 3 (Fig. 3). Surface water layers (0–2.5 m) were well oxygenated, while the deeper layer (15–80 m) were poorly oxygenated (oxygen saturation > 90% and 1.4–6.9%, respectively). The concentrations of DO and oxygen saturation was positively correlated with pH (Table 2).
The EC values (58–215 mS cm− 1) indicated high mineral salt concentrations in the pit lake water. The concentrations of major anions and cations in the water were in the following ranges (in g dm− 3): 19–167 for Cl−, 5.4–78.6 for SO42−, 0.3–6.1 for HCO3−, 15.2–125.3 for Na+, 2.0–35.3 for K+, not detected (nd)–0.6 for Ca2+ and 1.3–27.8 for Mg2+. Vertical profiles of Cl−, SO42−, Na+, K+ and Mg2+ concentrations in the pit lake water were similar (Fig. 3). They were lower in the surface water layer (0–5 m) and then gradually increased towards the bottom. They were similar or lower at the depth of 0–30 m, while below 30 m towards the bottom, they were higher in November compared to July. In July, ion concentrations showed a more irregular pattern below a depth of 40 m compared to November. Correlation coefficients between these ions confirm their interrelationship in the water column (Table 2) and their higher concentrations in November than in July (Table 1). Concentrations of Ca2+ and HCO3− showed considerable variability (Fig. 3). We found a positive correlation between HCO3− and Ca2+ (Table 2).
The pit lake water was highly mineralized (expressed as dry mass, 44.7–420.0 g dm− 3; Fig. 3). Mineralization increased with pit lake depth, reaching its highest values in the near bottom water in July and November, which was seven- and nine-fold higher, respectively, than the surface water. Concentration of dry mass showed a negative correlation with pH and DO and was positively correlated with major anions and cations, especially Cl− and Na+, and metals (Table 2).
Concentrations of nutrients in the pit lake water were high (nd–413 mg dm− 3 for NO3−, nd–39.3 mg dm− 3 NH4+ and nd–210.6 mg dm− 3 PO43−). Nutrients showed vertical variation (Fig. 3), lower nitrate concentrations were found in deep waters and the highest concentrations were at depths of 50 m in July and 15 m in November. The NH4+ levels showed an irregular vertical pattern in July, with the highest value at 15 m and the lowest values at the surface and at 50 m. In November, NH4+ levels increased from a depth of 15 m towards the bottom. Phosphates concentrations were greater in deeper water, with the peak concentration in the near-bottom water in July. Phosphate concentrations was higher in July than in November (Table 1). Nutrient concentrations were not correlated with other physicochemical parameters.
Metals
The metal concentrations in the lake water were in the following ranges: 148–1485 µg dm− 3 for Cd, 0.4–7.3 mg dm− 3 for Pb, 79–760 µg dm− 3 for Cu, 99–2040 µg dm− 3 for Zn, 1.7–11.0 mg dm− 3 for Mn, 0.4–5.9 mg dm− 3 for Fe, and 0.4–4.0 mg dm− 3 for Ni. Metal concentrations were lower in the surface water in July and at depths of 0–5 m in November (Fig. 4). In the deeper layers, metal concentrations (with the exception of Fe) were rather similar in July, but gradually increased in November. The concentrations of Cd, Pb, Cu, and Ni showed little variability from a depth of 50 m towards the bottom. Most metals decreased in the near-bottom water.
Metal concentrations were negatively correlated with pH (with the exception of Zn), DO, and oxygen saturation (with the exception of Mn and Fe; Table 2) and positively correlated with Cl−, SO42−, Na+, K+, and Mg2+. The strongest correlations were between Cd, Pb, Cu, Ni, Cl−, and SO42− and between Fe and Mn and Cl− and SO42−. The concentrations of Cd, Cu, Zn, Mn, and Ni were higher in November than in May (Table 1).
Hydrocarbons
We only found trace amounts of hydrocarbons, with no major peaks in the range of the light hydrocarbons < nC10. The retention was analysed for the range defined by the boiling point of n-paraffinic hydrocarbons nC10–nC36. Within this range, three peaks with a retention time characteristic of C21, C26, and C29 appeared in a sample from a depth of 85 m. The sample from 50 m showed a peak of an unidentified hydrocarbon between a retention time of C11 and C12. Subsequent peaks were assigned to C15 and C21, while an unidentified hydrocarbon had a retention time typical of C23 and C24 (Fig. 5, left-hand graph).
The boiling point of the substance was close to that of n-paraffinic hydrocarbons (Fig. 5). Other alkanes were present, albeit in small amounts. The concentration of alkane (paraffin) hydrocarbons was low, < 0.1 mg dm− 3 near the bottom (85 m) and higher, 0.2 mg dm− 3 at a depth of 50 m.
Biota
In the non-preserved samples, we observed a rich population of small nanoplanktonic heterotrophic flagellates, with a diameter of about 2 µm, and unicellular algae. Analysis of chlorophyll concentrations confirmed the presence of algae from various systematic taxa (Table 3). Chlorophylls were present in low concentrations throughout the lake beyond the light transmission range. Besides flagellates, live ciliates and one rotifer species were observed. Single aphids and Acarina were found. Among autotrophic seston organisms, some Bacillariophyta and Ceratium sp. were noted, as well as plant tracheas and their pollens. In June, we registered the live rotifer Brachionus plicatilis and two species of ciliates, Paradileptus elephantinus and Tindinnidium sp. In addition to the living organisms, we found a few dead rotifers, cladocerans and copepods, 19 species in total (Table 4). In autumn, the only live species was B. plicatilis, and Protozoa were absent. Including dead organisms, we noted a total of 10 species (Table 5).
The occurrence of Tintinnidium sp. was limited to the euphotic zone (to a depth of 2.5 m). Paradileptus elephantinus was most numerous at a depth of 5 m and penetrated the water layer up to a depth of 40 m into anoxic environments. Brachionus plicatilis was observed throughout the water profile with a maximum density at a depth of 7.5 m in June. In autumn, B. plicatilis was observed in the upper 20 m layer with a maximum density at a depth of 20 m (Tables 4, 5).
The analysed benthic sample comprised several diatom taxa that are known to occur in inland saline spring water (Wojtal 2013) and other athalassic habitats (Żelazna-Wieczorek et al. 2015). The material was dominated by Nitzschia pusilla and some Halamphora species (H. borealis, H. tenerrima, H acutiuscula). We also identified Navicula cincta, N. phylleptostoma, N. salinicola, Navicymbella pusilla, Nitzschia epithemoides, N. hungarica, N. scalpeliformis, and Staurophora lanceolata.
Discussion
According to the classification of mine water quality (Pluta and Grmela 2006), the upper layer (surface 5–10 cm in May, and 0–5 m in November) of the pit lake was classified as moderately saline, and the deeper layer as brine. Moderately saline water is characterized by a dry residue ranging from 3 to 70 g dm− 3 and concentrations of Cl− and SO42− from 1.8 to 42 g dm− 3, while brine is characterized by a dry residue > 70 g dm− 3 and concentrations of Cl− and SO42− > 42 g dm− 3 (Pluta and Grmela 2006). For biological purposes, Hammer’s classification is often used. According to Hammer (1993), the surface 5–10 cm layer belongs to the mesohaline type (20–50 g dm− 3), whereas the lower layer is hypersaline sensu Hammer, > 50 g dm− 3. The concentrations of anions and cations (with the exception of Ca2+) in the brine greatly exceeded the concentrations in sea water (Kabata-Pendias and Pendias 1999). The high levels of Na–Cl in the brine is presumably due to dissolution of halite from the slopes. The Cl− concentrations in the lake are similar to those in highly mineralized water bodies such as the Dead Sea, which has the characteristics of Ca–Cl brine (Gavrieli et al. 2001). The meromixis of the studied pit lake is classified as ectogenic, in contrast to biogenic and crenogenic meromixis (Boehrer and Schulze 2006).
Analysis of the pit lake’s chemistry shows the formation of two layers with different levels of mineralization and a seasonal increase in the mesohaline layer. Although the final salinity of the upper layer, when the lake chemistry stabilizes after the pit fills in 2025, is still unknown, we found it to be less mineralized (44.7 g dm− 3 in November) than predicted by Dolin et al. (2010). Our results align more with Gajdin et al. (2014) and Gaydin and Diakiv (2010), who predicted the ultimate occurrence of a freshwater surface layer. We observed decreasing salinity, from 179.1 to 44.7 g dm− 3, from a depth of 10 m to the near-surface in November, indicating a diminishing rate of salinity with decreasing depth of 13.4 g dm− 3 m− 1. Using the linear rate of salinity decrease, we predict that the pit lake may have layer of freshwater containing ≈ 1 g dm− 3 of dry residue at an elevation of 279 m a.s.l. (i.e. 4 m above the current lake level, with the pit filling at a rate of about 4 m per year). However, confirmation of our prognosis requires future validation.
Lakes like Lake Mogilnoe on Kildin Island in the Barents Sea (Fokin et al. 2004; Strelkov et al. 2014) and the Big Soda Lake in the USA (Cloern et al. 1983) (Fig. 6), demonstrate that surface water layers may not have a salinity < 3 g dm− 3, but may instead be hyposaline sensu Hammer (1993; 3–20 g dm− 3). The Mogilnoe Lake on Kildin Island on the Kola Peninsula in Russia was created as a separate bay of the Barents Sea about 1000 years ago. Currently, it has a depth of 17 m and is separated from the sea by a 3.7–5.4 m natural dike with a width of 63–70 m. The Mogilnoe Lake is hyposaline sensu Hammer (1993) in the surface layer and saline below. Another example is the the Kislo-Sladkoe Lake (Krasnowa and Pantyulin 2013; Krasnowa et al. 2014), which has layers of fresh, brackish (1000–4000 g dm− 3), and salty water with hydrogen sulphide.
In the Dombrovska pit lake, the similarity in the vertical distribution of Cl−, SO42−, Na+, K+, Mg2+, and metals may indicate similar mineralogical (or geological) sources and geochemical pathways for metals and ions. Some irregularities in the Cl−, SO42−, Na+, K+, Mg2+ concentrations in brine in the Dombrovska pit lake may be related to biogeochemical processes in the water column, chemical composition of groundwater supplying the pit lake, leaching of elements from easily soluble salts created by slopes and/or mixing of water. The Dombrovska pit lake is not yet completely filled with water; therefore, the freshwater inflow and mixing of freshwater and brine may still further modify water quality and the spatial distribution of elements in the water column. As the banks/slopes of the opencast had not been covered by any material before filling with water, minerals are continuously leached, this may lead to an increase in the concentration of some ions at certain depths.
The Dombrovska pit water is heavily contaminated. Concentrations of Fe are an order of magnitude greater, Cu, Zn, Mn, and Ni two orders of magnitude greater, and Cd and Pb three orders of magnitude greater than those found in less polluted water (Karnaukhova 2008; Szarek-Gwiazda and Mazurkiewicz-Boroń 2006). The Pearson’s correlation indicates that the geochemical pathway of metals in the lake water is affected by inorganic ligands (chlorides and sulphides). The ligands keep the metals in solution and influence their vertical variation. Interdependency of metals with strong inorganic ligands is often observed in meromictic lakes (Szarek-Gwiazda and Żurek 2006; Taillefert and Gaillard 2002).
The decreased concentrations of Cd, Pb, Zn, Mn, and Ni, and SO42− in the near-bottom water is probably due to sulphate reduction and precipitation of metal sulphides. This has been observed in other meromictic reservoirs and lakes (Balistrieri et al. 1994; Szarek-Gwiazda and Żurek 2006). Inversely, increased concentrations of Fe and PO43− in the near-bottom water of the Dombrovska pit lake in July indicate their release from the sediment. The amount of Fe and phosphorus released from the sediment and interstitial water to the near-bottom water depends on both Eh and pH. At neutral pH, the solubility of FePO4 is about 450 mg dm− 3 and increases at a pH > 7.5 (Golubiev and Savenko 2002). Measured Fe and phosphate concentrations did not exceed the oversaturation threshold for FePO4 precipitation. Hydrous Fe and Mn oxides are important scavengers of metals in various aquatic ecosystems; therefore, metal distribution in the water column of lakes and reservoirs is often related to Fe cycling (Szarek-Gwiazda and Żurek 2006; Taillefert and Gaillard 2002). Positive correlations between Mn, Fe, Cd, Pb, Cu, Zn, and Ni indicate that Fe and Mn oxides/hydroxides play an important role in the geochemical pathway of other metals in the lake.
The Dombrovska pit lake has a thermal profile totally different from those of other freshwater lakes. The temperature of the less mineralized water (depth 0–5 m) changes seasonally and is higher in July than in November. In July, because the period of high air temperatures is relatively short and the surface water absorbs most of the solar radiation, the temperature varies from about 22 °C at the surface to 16 °C at a depth of 5 m. From a depth of about 10 m to the bottom, there is no seasonal variation in temperature, which was found to be almost a constant (18 °C), despite an air temperature of less than 0 °C in November. Because brine is denser than the upper brackish water, the warm water does not rise. This phenomenon was also observed in the hypersaline, permanently ice-covered Vanda Lake (depth 68 m) in the Antarctic (Wilson and Wellman 1962). There, the ice cover had a thickness of 3.5 m and the air temperature was − 40 °C. The temperature of Vanda Lake water was 19.2 °C at a depth of 57.5 m. This “anomaly” is a result of heat trapped in saline lakes, which act as solar collectors. A detailed description of this heat trap mechanism and the physical processes of a solar pond are given by Hull (1979) and Weinberger (1964).
The low concentrations of hydrocarbons found at depths of 50 and 83 m may be explained in two ways: hydrocarbons released from included oil fluid in salt minerals, and pollution, possibly due to ore exploitation and transport. Light fractions of anthropogenic hydrocarbons evaporate or float up during flooding, and heavy fractions concentrate at the bottom or are gravitationally fractionated, depending on their density. Since the clay minerals at the pit base (50–83 m) contain heavy hydrocarbon fractions, the anthropogenic origin of the hydrocarbons is more probable.
In the shallow mesohaline water layer, only three live species were found: the rotifer Brachionus plicatilis and two Ciliata taxa: Paradileptus elephantinus and Tindinnidium sp. Such high salt concentrations can be toxic for water organisms (Cowgill and Milazzo 1990; Kipriyanova et al. 2007). Most macroinvertebrates are able to live at salinity levels < 2 g dm− 3, but for some macroinvertebrates, the salinity threshold is below 1 g dm− 3. In nature, increased salinity levels between 1 and 5 g dm− 3 cause a reduction in both species richness and abundance of zooplankton. Similar results were found by Kipriyanova et al. (2007) who noticed a decrease in species richness of zooplankton from 61 to 16 species as salinity increased from 0.8 to 6.4 g dm− 3. The hatching of resting eggs was inhibited at higher salinity levels between 16 and 32 g dm− 3. However, some resting eggs remained viable and hatched when being returned to freshwater (Santangelo et al. 2014). Also, KCl is much more toxic than NaCl or CaCl to zooplankton and fish (Doudoroff and Katz 1953).
Experimental data show that acute toxicity (as LC50) for freshwater cladocerans occurred below 3 g dm− 3 of NaCl; however, for Daphnia magna, toxicity was observed at various concentrations: 6.7 g dm− 3 by Cowgill and Milazzo (1990), 3.68 g dm− 3 by Anderson et al. (1948), and 4.625 g dm− 3 by Biesinger and Christensen (1972). The 96 h LC50 for caddisfly, mayfly, and midge ranged between 3.5 and 6 g dm− 3; other mayfly species are usually more sensitive (< 0.7 g dm− 3). Anderson et al. (1948) reported a 64 h immobilisation threshold for Cyclops vernalis, Mesocyclops leuckartii, Diaptomus oregonensis, Leptodora kindti, and D. magna in the range of 0.127–0.640 g dm− 3 of KCl and 3.030–6.100 g dm− 3 of NaCl. However, laboratory toxicity tests results are often inconsistent with field observations. For example, Anufriieva et al. (2014) evaluated hypersaline water bodies from the Crimean Peninsula and reported four Copepoda: Acanthocyclops sp. at a salinity of 210 g dm− 3; Eucyclops sp. copepodit at 150 g dm− 3; Diacyclops bisetosus and Cyclops furcifer at 140–150 g dm− 3 (Table 6). It is likely that some freshwater species are able to adapt to higher salinity levels in natural environments.
The occurrence of Paradileptus elephantinus and Tindinnidium sp. in the deeper hypersaline layer of the Dombrovska pit lake were unexpected (Table 5). We therefore considered that these species had adapted to the hypersaline water or sank from the upper layer into deeper layers, if sinking is possible in this dense hypersaline water. The specific density (measured aerometrically) of the pit lake water is high, from 1.032 g cm− 3 at the surface to 1.113 g cm− 3 at a depth of 10 m and to 1.282 g cm− 3 in the near-bottom water (85 m) (unpublished data). The specific gravity of chitin is between 1.39 and 1.41 g cm− 3 (Sollas 1907). Therefore sinking of chitin in the salt water is possible. The dead planktonic animals we observed were probably washed in from surrounding freshwater bodies and died of osmotic shock on contact with the saline pit lake water. In this sense, this saline lake might be a trap for freshwater animals.
Samples of the lake water were rich in diatoms and had a similar taxa composition as other saline springs (Wojtal 2013; Żelazna-Wieczorek et al. 2015). The diatoms found have also been observed in water with very high EC values and with high amounts of calcium, magnesium, and other cations (Wojtal 2013). Diatoms can inhabit waters with extremely low oxygen levels. Several taxa are exclusive to oxygen-poor water (e.g. Halamphora borealis, H. tenerrima, H. acutiuscula, Navicula phylleptostoma, N. salinicola, or Staurophora lanceolata), which is related to their ability to inhabit highly saline inland waters. Moreover, Halamphora tenerrima is known from marine environments (Żelazna-Wieczorek et al. 2015). Species such as Navicula cincta, Navicymbula pusilla, Nitzschia hungarica, and N. pusilla are widely distributed in waters rich in dissolved minerals (Krammer and Lange-Bertalot 1986, 1988; Wojtal 2013; Żelazna-Wieczorek et al. 2015), including those rich in metal ions. The diatoms, which have rarely been reported in water with high EC levels, were also relatively rare species here.
Conclusions
The high mineralization of the ectogenic Dombrovska pit lake water creates a remarkable aquatic environment. The pit lake is not yet completely filled with water and the water quality has not reached its final state, due to the inflow and mixing of freshwater and brine. In contrast to typical meromictic lakes, which are usually divided into three layers (mixolimnion, monimolimnion, and chemocline), the Dombrovska pit lake has only two distinct layers: a thick (ca. 10 m) upper mesohaline, oxic layer, and a large (ca. 75 m) anoxic hypersaline layer (monimolimnion), rich in major anions and cations (except for Ca2+), metals, and nutrients. The two layers do not mix and in this sense, the Dombrovska pit lake is fairly unique. The directions of changes in water chemistry tend to confirm our hypothesis that, after fully filling the void, the pit lake will have a freshwater or brackish water upper layer and a lower, saline monimolimnion. The change from present lake status to the meromictic lake type is predicted and will involve the gradual increase in thickness of the upper, oxic (mixolimnion) layer. The deep lower, anoxic layer, rich in ions and metals will remain but will be less thick. We were unable, based on current data, to predict the final salinity of the upper layer, whether it will be fresh or brackish water. We predict that in the future, a “true” meromic pit lake with classical chemical stratification in the water column will be formed. However, our prognosis requires verification in further studies.
High water mineralization and concentrations of metals creates unsuitable conditions for most biota. In terms of zooplankton, only three living taxa were found: the rotifer Brachionus plicatilis and two species of ciliates, Paradileptus elephantinus and Tindinnidium. In the littoral part of the pit lake, we found diatoms resistant to high salinity, such as Nitzschia pusilla and some Halamphora species (H. borealis, H. tenerrima, and H. acutiuscula). Most freshwater species live in waters with a salinity of 1 g dm− 3 or a little more. With increasing salinity, brackish water species appear. The conspicuous feature of the diatom assemblage is the lack of a widespread, non-saline taxa. As the concentration of ions decreases, taxa with a wide spectrum of tolerance for environmental conditions may appear. As the lake is young and still filling, its chemistry and succession of living organisms are expected to be subject to considerable fluctuations, and additional investigations.
References
Anderson BG (1948) The apparent thresholds of toxicity to Daphnia magna for chlorides of various metals when added to Lake Erie water. Trans Am Fish Soc 98:96–113
Anufriieva E, Hołyńska M, Shadrin N (2014) Current invasions of Asian cyclopid species (Copepoda: Cyclopidae) in Crimea, with taxonomical and zoogeographical remarks on the hypersaline and freshwater fauna. Ann Zool 64(1):109–130
Balistrieri LS, Murray JW, Paul B (1994) The geochemical cycling of trace elements in abiogenic meromictic lake. Geochim Cosmochim Acta 58(19):3993–4008
Bielańska-Grajner I, Ejsmont-Karabin J, Iakovenko N (2014) Wrotki Rotifera Bdelloidea Wydawnictwo Uniwersytetu Łódzkiego, Ser. Fauna Słodkowodna Polski, Łódź, pp 1–128
Biesinger KE, Christensen GM (1972) Effects of various metals on survival, growth, reproduction, and metabolism of Daphnia magna. J Fish Res Board Can 29:1691–1700
Boehrer B, Schultze M (2006) On the relevance of meromixis in mine pit lakes. In: Barnhisel RI (ed) Proceedings 7th international conference on acid rock drainage (ICARD), American Society of Mining and Reclamation, pp 200–213
Brucet S, Boix D, Gascón S, Sala J, Quintana XD, Badosa A, Søndergaard M, Lauridsen TL, Jeppesen E (2009) Species richness of crustacean zooplankton and trophic structure of brackish lagoons in contrasting climate zones: north temperate Denmark and Mediterranean Catalonia (Spain). Ecography 32:692–702
Carrasco NK, Perissinotto R (2012) Development of a halotolerant community in the St. Lucia Estuary (South Africa) during a hypersaline phase. PLoS One 7(1):e29927. https://doi.org/10.1371/journal.pone.0029927
Cloern JE, Cole BE, Oremland RS (1983) Seasonal changes in the chemistry and biology of a meromictic lake. (Big Soda Lake, Nevada, USA). Hydrobiologia 105:195–206
Cowgill UM, Milazzo DP (1990) The sensitivity of two Cladocerans to water quality variables: salinity and hardness. Arch Hydrobiol 120:185–196
Del Ponti OD, Cabrera GC, Vignatti AM, Echaniz SA (2015) Dynamics of the limnological parameters and zooplankton of La Brava, a shallow lake of the Atuel-Salado-Chadileuvú-Curacó Rivers system (La Pampa, Argentina). App Ecol Environ Sci 3(6):193–199
Dolin VV, Yakovlev EO, Kuzienko ED, Baraninko BT (2010) Prediction eko-hydro-chemical situation with flooding Dombrowski potash ores open pit. Nauk-Tekhn Zh 1:74–87 (in Ukranian)
Doudoroff P, Katz M (1953) Critical review of literature on the toxicity of industrial wastes and their components to fish. Sew Ind Wastes 25:802–839
Dussart B (1969) Les copépodes des eaux continentales d’europe occidentale. T. II. Cyclopoïdes et biologie. Boubée, Paris
Flössner D (1972) Kiemen-und Blattfüsser, Branchiopoda, Fischläuse, Branchiura. In: Dahl M, Peus F (eds), Die Tierwelt Deutschlands 60. Gustav Fischer Verlag, Jena, pp 1–499
Foissner W, Berger H (1996) A user-friendly guide to the ciliates (Protozoa, Ciliophora) commonly used by hydrobiologists as bioindicators in rivers, lakes, and waste waters, with notes on their ecology. Freshw Biol 35(2):375–482
Foissner W, Berger H, Schaumburg J (1999) Identification and ecology of limnetic plankton ciliates, Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft, München
Fokin MV, Shunatova NN, Usov NV, Bufalova EN, Malavenda SS, Red’kin DV, Strelkov PP, Shoshina EV (2004) Relick Lake Mogilnoe-2003. Scientific report of the expedition of the SPbGU-MSTU-ZINRANA on the insland of Kildin, Lake Mogilnoe. St. Petersburg, pp 20
Gajdin AM, Maciaszek J, Szewczyk J (2014) The influence of the inundation of the potassium open pit in Kalush on the environment-predictions and facts. GaEE 8:15–24
Gavrieli I, Yechieli Y, Halicz L, Spiro B, Bein A, Efron D (2001) The sulfur system in anoxic subsurface brines and its implication in brine evolutionary pathways: the Ca–chloride brines in the Dead Sea area. Earth Planet Sci Lett 186(2):199–213
Gaydin AM (2011) Lake in potash-salt open pit of the Dombrovo. Sci Tech J Ecol Safe Bal Resour Use 2(4):55–62
Gaydin AM, Dyakiv VO (2010) Conditions forming of freshwater layer are in a Lake on place salt quarry. Pryroda Zakhidnoho Polissya ta Prylehlykh terytoriy 7:50–64 (in Ukranian)
Gilabert J (2001) Seasonal plankton dynamics in a Mediterranean hypersaline coastal lagoon: the Mar Menor. J Plank Res 23:207–218
Golubiev SV, Savenko AV (2002) Solubility of iron(III) and aluminium phosphates in aqueous solutions. Goldschmidt Conf Abstracts, p A253
Hammer UT (1993) Zooplankton distribution and abundance in saline lakes of Alberta and Saskatchewan, Canada. Int J Salt Lake Res 2(2):111–132
Hammer UT (1994) Life and times of five Saskatchewan saline meromictic lakes. Hydrobiology 79:235–248
Hull JR (1979) Physics of the solar pond. Retrospective Theses and Dissertations Paper 6608
Kabata-Pendias A, Pendias H (1999) Trace metals biogeochemistry. PWN, Warszawa
Karnaukhova GA (2008) Hydrochemistry of the Angara and reservoirs of the Angara cascade. Water Res 35:71–79
Kipriyanova L, Yermolaeva N, Bezmaternykh D, Dvurechenskaya S, Mitrofanova E (2007) Changes in the biota of Chany Lake along a salinity gradient. Hydrobiologia 576(1):83–93
Koste W (1978) Rotatoria. Die Rädertiere Mitteleuropas begründet von Max Voigt. Berlin, Stuttgart
Krammer K, Lange-Bertalot H (1986) Bacillariophyceae. Naviculaceae. In: Ettl G, Gerloff J, Heynig H, Mollenhauer D (eds), Süβwasserflora von Mitteleuropa 2/1. VEB Gustav Fischer Verlag, Jena
Krammer K, Lange-Bertalot H (1988) Bacillariophyceae. Bacillariaceae, Epithemiaceae, Surirellaceae. In: Ettl G, Gerloff J, Heynig H, Mollenhauer D (eds) Süβwasserflora von Mitteleuropa 2/2. G. Fischer Verlag, Stuttgart
Krammer K, Lange-Bertalot H (1991) Bacillariophyceae. Centrales, Fragilariaceae, Eunotiaceae. In: Ettl G, Gerloff J, Heynig H, Mollenhauer D (eds) Süβwasserflora von Mitteleuropa 2/3. Fischer Verlag, Stuttgart
Krammer K, Lange-Bertalot H (2004) Achnanthaceae, Kritische Erganzungen zu Navicula (Lineolatae) und Gomphonema Gesamtliteraturverzeichnis Teil 1–4. In: Ettl G, Gerloff J, Heynig H, Mollenhauer D (eds), Süβwasserflora von Mitteleuropa 2/4. Gustav Fischer Verlag, Stuttgart
Krasnova ED, Pantyulin AN (2013) Belomorskiye fresh water-acidic lake, full of wonders. Pryroda 2:39–48
Krasnova ED, Pantyulin AN, Matorin DN, Todorenk DA, Belevich TA, Milyutina IA, Voronov DA (2014) Cryptomonad alga Rhodomonas sp. (Cryptophyta Pyrenomonadaceae) bloom in the redox zone of the basins separating from the White Sea. Microbiology 83(3):270–277
Mitchell BG, Kiefer DA (1984) Deterermination of absorption and fluorescence excitation spectra for phytoplankton. In: Holm-Hansen O, Bolis L, Giles R (eds) Marine phytoplankton and productivity. Springer, Berlin
Pluta I, Grmela A (2006) Odprowadzanie wód kopalnianych do Odry w świetle przepisów prawnych w Polsce i Republice Czeskiej [Discharge of mine water into the Oder in the light of legal regulations in Poland and the Czech Republic]. In: VI Konferencja naukowo-techniczna “Ochrona środowiska na terenach górniczych” [VI scientific and technical Conference “Environmental protection in mining areas”. AGH Kraków, PS Sosnowiec, GIG Sosnowiec. pp 377–392 (in Polish)
Rios P, Crespo JE (2004) Salinity effects on the abundance of Boeckella poopoensis (Copepoda, Calanoida) in saline ponds in the Atacama Desert, northern Chile. Crustaceana 77:417–423
Santangelo JM, Esteves FA, Manca M, Bozelli RL (2014) Disturbances due to increased salinity and the resilience of zooplankton communities: the potential role of the resting egg bank. Hydrobiologia 722:103–113
Sollas I (1907) On the identification of chitin by its physical constants. Proc R Soc B Biol Soc 534:474–481
Strelkov P, Shunatova N, Fokin M, Usov N, Fedyuk M, Malavenda S, Lubina O, Poloskin A, Korsun S (2014) Marine lake Mogilnoe (Kildin Island, the Barents Sea): one hundred years of solitude. Polar Biol 37:297–310
Szarek-Gwiazda E, Mazurkiewicz-Boroń G (2006) Influence of cadmium and lead partitioning in water and sediment on their deposition in the sediment of a eutrophic dam reservoir. Oceanol Hydrobiol Stud 35(2):141–157
Szarek-Gwiazda E, Żurek R (2006) Distribution of trace elements in meromictic pit lake. Water Air Soil Pollut 174:181–196
Taillefert M, Gaillard JF (2002) Reactive transport modeling of trace elements in the water column of a stratified lake: iron cycling and metal scavenging. J Hydrol 256:16–34
Weinberger H (1964) The physics of the solar pond. Sol Energy 8(2):45–56
Wilson AT, Wellman HW (1962) Lake Vanda: an Antarctic Lake: Lake Vanda as a solar energy trap. Nature 196:1171–1173
Wojtal AZ (2013) Species composition and distribution of diatom assemblages in spring waters from various geological formations in southern Poland. Bibliotheca Diatomologica 59:1–436 (Cramer J, Gebrüder Borntraeger Verlagsbuchhandlung, Stuttgart)
Żelazna-Wieczorek J, Olszyński RM, Nowicka-Krawczyk P (2015) Half a century of research on diatoms in athalassic habitats in central Poland. Oceanol Hydrobiol Stud 44(1):51–67
Żurek R (2006) Chemical properties of water in a flooded opencast sulphur mine. Aquat Ecol 40:135–153
Acknowledgements
The study was partially financed by the Institute of Nature Conservation PAS, Krakow.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Żurek, R., Diakiv, V., Szarek-Gwiazda, E. et al. Unique Pit Lake Created in an Opencast Potassium Salt Mine (Dombrovska Pit Lake in Kalush, Ukraine). Mine Water Environ 37, 456–469 (2018). https://doi.org/10.1007/s10230-018-0527-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-018-0527-z