Abstract
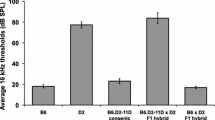
The 129S6/SvEvTac (129S6) inbred mouse is known for its resistance to noise-induced hearing loss (NIHL). However, less is understood of its unique age-related hearing loss (AHL) phenotype and its potential relationship with the resistance to NIHL. Here, we studied the physiological characteristics of hearing loss in 129S6 and asked if noise resistance (NR) and AHL are genetically linked to the same chromosomal region. We used auditory brainstem response (ABR) and distortion product otoacoustic emissions (DPOAE) to examine hearing sensitivity between 1 and 13 months of age of recombinant-inbred (congenic) mice with an NR phenotype. We identified a region of proximal chromosome (Chr) 17 (D17Mit143-D17Mit100) that contributes to a sensory, non-progressive hearing loss (NPHL) affecting exclusively the high-frequencies (>24 kHz) and maps to the nr1 locus on Chr 17. ABR experiments showed that 129S6 and CBA/CaJ F1 (CBACa) hybrid mice exhibit normal hearing, indicating that the hearing loss in 129S6 mice is inherited recessively. An allelic complementation test between the 129S6 and 101/H (101H) strains did not rescue hearing loss, suggesting genetic allelism between the nphl and phl1 loci of these strains, respectively. The hybrids had a milder hearing loss than either parental strain, which indicate a possible interaction with other genes in the mouse background or a digenic interaction between different genes that reside in the same genomic region. Our study defines a locus for nphl on Chr 17 affecting frequencies greater than 24 kHz.









Similar content being viewed by others
References
Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MF, Fisher EM (2000) Genealogies of mouse inbred strains. Nat Genet 24:23–26
Charizopoulou N, Lelli A, Schraders M, Ray K, Hildebrand MS, Ramesh A, Srisailapathy CR, Oostrik J, Admiraal RJ, Neely HR, Latoche JR, Smith RJ, Northup JK, Kremer H, Holt JR, Noben-Trauth K (2011) Gipc3 mutations associated with audiogenic seizures and sensorineural hearing loss in mouse and human. Nat Commun 2:201
Davis RR, Newlander JK, Ling X, Cortopassi GA, Krieg EF, Erway LC (2001) Genetic basis for susceptibility to noise-induced hearing loss in mice. Hear Res 155:82–90
Dunn LC, Caspari E (1945) A case of neighboring loci with similar effects. Genetics 30:543–568
Erway LC, Willott JF, Archer JR, Harrison DE (1993) Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hear Res 65:125–132
Erway LC, Shiau YW, Davis RR, Krieg EF (1996) Genetics of age-related hearing loss in mice. III. Susceptibility of inbred and F1 hybrid strains to noise-induced hearing loss. Hear Res 93:181–187
Govaerts PJ, De Ceulaer G, Daemers K, Verhoeven K, Van Camp G, Schatteman I, Verstreken M, Willems PJ, Somers T, Offeciers FE (1998) A new autosomal-dominant locus (DFNA12) is responsible for a nonsyndromic, midfrequency, prelingual and nonprogressive sensorineural hearing loss. Am J Otol 19:718–723
Häfner FM, Salam AA, Linder TE, Balmer D, Baumer A, Schinzel AA, Spillmann T, Leal SM (2000) A novel locus (DFNA24) for prelingual nonprogressive autosomal dominant nonsyndromic hearing loss maps to 4q35-qter in a large Swiss German kindred. Am J Hum Genet 66:1437–1442
Henderson D, Bielefeld EC, Harris KC, Hu BH (2006) The role of oxidative stress in noise-induced hearing loss. Ear Hear 27:1–19
Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY (1997) A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res 114:83–92
Johnson KR, Zheng QY, Erway LC (2000) A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics 70:171–180
Kane KL, Longo-Guess CM, Gagnon LH, Ding D, Salvi RJ, Johnson KR (2012) Genetic background effects on age-related hearing loss associated with Cdh23 variants in mice. Hear Res 283:80–88
Keithley EM, Canto C, Zheng QY, Fischel-Ghodsian N, Johnson KR (2004) Age-related hearing loss and the ahl locus in mice. Hear Res 188:21–28
Keller JM, Noben-Trauth K (2012) Genome-wide linkage analyses identify Hfhl1 and Hfhl3 with frequency-specific effects on the hearing spectrum of NIH Swiss mice. BMC Genet 13:32
Keller JM, Neely HR, Latoche JR, Noben-Trauth K (2011) High-frequency sensorineural hearing loss and its underlying genetics (Hfhl1 and Hfhl2) in NIH Swiss mice. J Assoc Res Otolaryngol 12:617–631
Kirschhofer K, Kenyon JB, Hoover DM, Franz P, Weipoltshammer K, Wachtler F, Kimberling WJ (1998) Autosomal-dominant, prelingual, nonprogressive sensorineural hearing loss: localization of the gene (DFNA8) to chromosome 11q by linkage in an Austrian family. Cytogenet Cell Genet 82:126–130
Kiss PJ, Knisz J, Zhang Y, Baltrusaitis J, Sigmund CD, Thalmann R, Smith RJ, Verpy E, Bánfi B (2006) Inactivation of NADPH oxidase organizer 1 results in severe imbalance. Curr Biol 16:208–213
Kitajiri S, Miyamoto T, Mineharu A, Sonoda N, Furuse K, Hata M, Sasaki H, Mori Y, Kubota T, Ito J, Furuse M, Tsukita S (2004a) Compartmentalization established by claudin-11-based tight junctions in stria vascularis is required for hearing through generation of endocochlear potential. J Cell Sci 117:5087–5096
Kitajiri SI, Furuse M, Morita K, Saishin-Kiuchi Y, Kido H, Ito J, Tsukita S (2004b) Expression patterns of claudins, tight junction adhesion molecules, in the inner ear. Hear Res 187:25–34
Kujawa SG, Liberman MC (2006) Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci 26:2115–2123
Latoche JR, Neely HR, Noben-Trauth K (2011) Polygenic inheritance of sensorineural hearing loss (Snhl2, -3, and -4) and organ of Corti patterning defect in the ALR/LtJ mouse strain. Hear Res 275:150–159
Liu XZ, Yuan Y, Yan D, Ding EH, Ouyang XM, Fei Y, Tang W, Yuan H, Chang Q, Du LL, Zhang X, Wang G, Ahmad S, Kang DY, Lin X, Dai P (2009) Digenic inheritance of non-syndromic deafness caused by mutations at the gap junction proteins Cx26 and Cx31. Hum Genet 125:53–62
Markand (1994) Brainstem auditory evoked potentials. J Clin Neurophysiol 11:320–342
Markel P, Shu P, Ebeling C, Carlson GA, Nagle DL, Smutko JS, Moore KJ (1997) Theoretical and empirical issues for marker-assisted breeding of congenic mouse strains. Nat Genet 17:280–284
Martin GK, Vázquez AE, Jimenez AM, Stagner BB, Howard MA, Lonsbury-Martin BL (2007) Comparison of distortion product otoacoustic emissions in 28 inbred strains of mice. Hear Res 234:59–7
Mashimo T, Erven AE, Spiden SL, Guenet JL, Steel KP (2006) Two quantitative trait loci affecting progressive hearing loss in 101/H mice. Mamm Genome 17:841–850
Moller MB (1994) Audiological evaluation. J Clin Neurophysiol 11:309–318
Morita Y, Hirokawa S, Kikkawa Y, Nomura T, Yonekawa H, Shiroishi T, Takahashi S, Kominami R (2007) Fine mapping of Ahl3 affecting both age-related and noise-induced hearing loss. Biochem Biophys Res Commun 355:117–121
Nakano Y, Kim SH, Kim HM, Sanneman JD, Zhang Y, Smith RJ, Marcus DC, Wangemann P, Nessler RA, Banfi B (2009) A claudin-9-based ion permeability barrier is essential for hearing. PLoS Genet 5:e1000610
Nemoto M, Morita Y, Mishima Y, Takahashi S, Nomura T, Ushiki T, Shiroishi T, Kikkawa Y, Yonekawa H, Kominami R (2004) Ahl3, a third locus on mouse chromosome 17 affecting age-related hearing loss. Biochem Biophys Res Commun 324:1283–1288
Noben-Trauth K, Zheng QY, Johnson KR (2003) Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet 35:21–23
Noben-Trauth K, Latoche JR, Neely HR, Bennett B (2010) Phenotype and genetics of progressive sensorineural hearing loss (Snhl1) in the LXS set of recombinant inbred strains of mice. PLoS One 5:e1145
Nunes FD, Lopez LN, Lin HW, Davies C, Azevedo RB, Gow A, Kachar B (2006) Distinct subdomain organization and molecular composition of a tight junction with adherens junction features. J Cell Sci 119:4819–4827
Ohlemiller KK, Gagnon PM (2004) Cellular correlates of progressive hearing loss in 129S6/SvEv mice. J Comp Neurol 469:377–390
Paffenholz R, Bergstrom RA, Pasutto F, Wabnitz P, Munroe RJ, Jagla W, Heinzmann U, Marquardt A, Bareiss A, Laufs J, Russ A, Stumm G, Schimenti JC, Bergstrom DE (2004) Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev 18:486–491
Pleis JR, Lethbridge-Cejku M (2007) Summary health statistics for U.S. adults: National Health Interview Survey, 2006. Vital Health Stat 10(235):1–153
Qin Z, Wood M, Rosowski JJ (2010) Measurement of conductive hearing loss in mice. Hear Res 263:93–103
Rosowski JJ, Brinsko KM, Tempel BL, Kujawa SG (2003) The aging of the middle ear in 129S6/SvEvTac and CBA/CaJ mice: measurements of umbo velocity, hearing function, and the incidence of pathology. J Assoc Res Otolaryngol 4:371–383
Sergeyenko Y, Lall K, Liberman MC, Kujawa SG (2013) Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci 33:13686–13694
Shin JB, Longo-Guess CM, Gagnon LH, Saylor KW, Dumont RA, Spinelli KJ, Pagana JM, Wilmarth PA, David LL, Gillespie PG, Johnson KR (2010) The R109H variant of fascin-2, a developmentally regulated actin crosslinker in hair-cell stereocilia, underlies early-onset hearing loss of DBA/2J mice. J Neurosci 30:9683–9694
Street VA, Kujawa SG, Manichaikul A, Broman KW, Kallman JC, Shilling DJ, Iwata AJ, Robinson LC, Robbins CA, Li J, Liberman MC, Tempel BL (2014) Resistance to noise-induced hearing loss in 129S6 and MOLF mice: identification of independent, overlapping, and interacting chromosomal regions. J Assoc Res Otolaryngol 15(5):721–738
Tremblay KL, Burkard RF (2007) The aging auditory system: confounding effects of hearing loss on AEPs. In: Burkard RF, Eggermont JJ, Don M (eds) Auditory evoked potentials: basic principles and clinical application. Lippincott Williams & Wilkins, Baltimore, pp 403–425
Vázquez AE, Jimenez AM, Martin GK, Luebke AE, Lonsbury-Martin BL (2004) Evaluating cochlear function and the effects of noise exposure in the B6.CAST + Ahl mouse with distortion product otoacoustic emissions. Hear Res 194:87–96
Witmer PD, Doheny KF, Adams MK, Boehm CD, Dizon JS, Goldstein JL, Templeton TM, Wheaton AM, Dong PN, Pugh EW, Nussbaum RL, Hunter K, Kelmenson JA, Rowe LB, Brownstein MJ (2003) The development of a highly informative mouse simple sequence length polymorphism (SSLP) marker set and construction of a mouse family tree using parsimony analysis. Genome Res 13:485–491
Yoshida N, Hequembourg SJ, Atencio CA, Rosowski JJ, Liberman MC (2000) Acoustic injury in mice: 129/SvEv is exceptionally resistant to noise-induced hearing loss. Hear Res 141:97–106
Zanchetta S, Ohara K, Rodrigues PT, Carvalho EL, Richieri-Costa A (2000) “New” autosomal-dominant infantile sensorineural non-progressive high-frequency hearing loss: report on a Brazilian family. Am J Med Genet 95:13–16
Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F (1997) The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90:181–192
Zheng QY, Johnson KR, Erway LC (1999) Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res 130:94–107
Zheng QY, Yan D, Ouyang XM, Du LL, Yu H, Chang B, Johnson KR, Liu XZ (2005) Digenic inheritance of deafness caused by mutations in genes encoding cadherin 23 and protocadherin 15 in mice and humans. Hum Mol Genet 14:103–111
Acknowledgments
The authors would like to thank Jennifer Larson for her constructive criticisms in the preparation of the manuscript, Sharon G. Kujawa for her support and discussion of techniques and results, Tina Chan for the ABR threshold verification, and the members of the Tempel laboratory for their technical support and constructive discussion of the research. This work was supported by grants from DoD W81XWH-11-2-0037 (BLT) and NIH R01DC02739 (BLT), R01DC06305 (BLT), P30DC04661 (BLT), T32DC000033 (BP), T32GM007108 (BP), and T32DC000018 (BP).
Conflict of Interest
The authors would like to declare no commercial or other conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peguero, B., Tempel, B.L. A Chromosome 17 Locus Engenders Frequency-Specific Non-Progressive Hearing Loss that Contributes to Age-Related Hearing Loss in Mice. JARO 16, 459–471 (2015). https://doi.org/10.1007/s10162-015-0519-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-015-0519-7