Abstract
Aminoglycoside ototoxicity involves the accumulation of antibiotic molecules in the inner ear hair cells and the subsequent degeneration of these cells. The exact route of entry of aminoglycosides into the hair cells in vivo is still unknown. Similar to other small organic cations, aminoglycosides could be brought into the cell by endocytosis or permeate through large non-selective cation channels, such as mechanotransduction channels or ATP-gated P2X channels. Here, we show that the aminoglycoside antibiotic gentamicin can enter mouse outer hair cells (OHCs) via TRPA1, non-selective cation channels activated by certain pungent compounds and by endogenous products of lipid peroxidation. Using conventional and perforated whole-cell patch clamp recordings, we found that application of TRPA1 agonists initiates inward current responses in wild-type OHCs, but not in OHCs of homozygous Trpa1 knockout mice. Similar responses consistent with the activation of non-selective cation channels were observed in heterologous cells transfected with mouse Trpa1. Upon brief activation with TRPA1 agonists, Trpa1-transfected cells become loaded with fluorescent gentamicin–Texas Red conjugate (GTTR). This uptake was not observed in mock-transfected or non-transfected cells. In mouse organ of Corti explants, TRPA1 activation resulted in the rapid entry of GTTR and another small cationic dye, FM1-43, in OHCs and some supporting cells, even when hair cell mechanotransduction was disrupted by pre-incubation in calcium-free solution. This TRPA1-mediated entry of GTTR and FM1-43 into OHCs was observed in wild-type but not in Trpa1 knockout mice and was not blocked by PPADS, a non-selective blocker of P2X channels. Notably, TRPA1 channels in mouse OHCs were activated by 4-hydroxynonenal, an endogenous molecule that is known to be generated during episodes of oxidative stress and accumulate in the cochlea after noise exposure. We concluded that TRPA1 channels may provide a novel pathway for the entry of aminoglycosides into OHCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aminoglycoside antibiotics are used worldwide despite their side effects (Forge and Schacht 2000). In hair cells of the inner ear, aminoglycosides can block mechanotransduction channels (Ohmori 1985; Kroese et al. 1989; Kros et al. 1992; Kimitsuki and Ohmori 1993; Ricci 2002; Marcotti et al. 2005) and nicotinic acetylcholine receptors (Blanchet et al. 2000). However, aminoglycoside ototoxicity develops following the entry of these molecules into the hair cells (Hiel et al. 1993), most likely due to their intracellular cytotoxic effects (Forge and Schacht 2000; Rizzi and Hirose 2007). One pathway for the entry of aminoglycosides into the cell could be endocytosis (Lim 1986; de Groot et al. 1990; Hashino and Shero 1995). Alternatively, these small cations could also permeate through non-selective cation channels such as the hair cell mechanotransduction channel that has a large pore of approximately 1.25 nm (Farris et al. 2004). Indeed, the rapid entry of these drugs into mammalian cochlear hair cells via mechanotransduction channels has been experimentally demonstrated (Marcotti et al. 2005; Luk et al. 2010).
The hair cell mechanotransduction channel has broad similarity to other non-selective cation channels, including the transient receptor potential (TRP) family of channels (Corey 2003; Strassmaier and Gillespie 2003; Christensen and Corey 2007). TRP channels function as chemoreceptors, thermoreceptors, osmoreceptors, and mechanosensors (for review, see Clapham 2003). Many members of the TRP family of channels are non-selectively permeable to cations and allow the entry of small organic molecules (Gale et al. 2001; Meyers et al. 2003; Corey et al. 2004; Banke et al. 2010; Karashima et al. 2010) including gentamicin (Myrdal et al. 2005). The TRPA1 channel, a member of the TRP family, may be expressed in cochlear hair cells (Corey et al. 2004), although its function in these cells is yet unknown (Bautista et al. 2006; Kwan et al. 2006). The pharmacological properties of TRPA1 channels are similar to that of mechanotransduction channels (Nagata et al. 2005). It was estimated that TRPA1 has a pore diameter between 1.1 and 1.38 nm (Karashima et al. 2010), which is large enough to allow permeation by aminoglycosides and other small organic cations.
Here, we demonstrate that cochlear outer hair cells (OHCs) possess functional TRPA1 channels and that aminoglycosides enter OHCs via these channels. We also show that TRPA1 channels in OHCs can be activated by 4-hydroxynonenal (4HNE), a thiol-reactive molecule (Macpherson et al. 2007b; Andersson et al. 2008) that is endogenously generated in the cochlea during episodes of oxidative stress, e.g., following intense noise exposure (Yamashita et al. 2004).
Methods
Organ of Corti explants
Mouse organ of Corti explants were prepared as previously described (Russell et al. 1986; Russell and Richardson 1987; Stepanyan and Frolenkov 2009). Explants were dissected at postnatal days 2–4 (P2–4) and placed in glass bottom Petri dishes (WillCo Wells, Amsterdam, the Netherlands). The organs of Corti were cultured at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) cell culture medium (Invitrogen, Carlsbad, CA) supplemented with 7% fetal bovine serum (FBS; Invitrogen) and 10 μg/ml of ampicillin (Calbiochem, La Jolla, CA). The explants were used in experiments within 1–5 days after dissection. All animal procedures were approved by the University of Kentucky Animal Care and Use Committee (protocol no. 903M2005).
Mouse genotypes
Trpa1 knockout mice were kindly provided to us by Drs. Kelvin Y. Kwan and David P. Corey. This strain is also known as B6;129P-Trpa1tm1Kykw/J (Jackson Laboratories, Bar Harbor, ME). In these mice, exons encoding the pore domain of TRPA1 were deleted (Kwan et al. 2006). Amplification of the transcript by RT-PCR indicated that Trpa1 message was present in wild-type and heterozygous animals, but absent in Trpa1 knockout mice, suggesting that there is no functional TRPA1 protein in knockout animals (Kwan et al. 2006). All Trpa1 knockout mice used in this study have been backcrossed to C57BL/6 mice (Jackson Laboratories) for at least six generations. Because heterozygous (Trpa1+/−) animals demonstrate an intermediate phenotype in a number of tests (Bautista et al. 2006; Kwan et al. 2006), we compared only wild-type (Trpa1+/+) and homozygous (Trpa−/−) mice in this study. To generate a sufficient quantity of these mice, we typically separated wild-type and homozygous sibling breeders from a Trpa1+/− × Trpa1+/− mating and subsequently mated Trpa1+/+ × Trpa1+/+ and Trpa1−/− × Trpa1−/− pairs. In few instances specifically indicated in the text, we used littermates from a Trpa1+/− × Trpa1+/− mating.
In order to genotype animals for their alleles of Trpa1, genomic DNA was extracted from tail snip samples and purified using a Wizard SV genomic purification kit (Promega Corporation, Madison, WI). Polymerase chain reaction (PCR) was performed using PCR Master Mix (Promega Corporation) as previously described (Kwan et al. 2006). Oligo primers no. 473 TCCTGCAAGGGTGATTGCGTTGTCTA and no. 474 TCATCTGGGCAACAATGTCACCTGCT were used to detect the wild-type allele, while oligo primers no. 517 ACTGAGCCCATGACACCAAACCT and no. 518 TGGACCTCTGATCCACTTTGCGTA were used to detect the mutant allele of Trpa1. PCR products were electrophoresed in a 1.5% agarose gel. Primers no. 473 and no. 474 produce a 300-bp band, while primers no. 517 and no. 518 produce a 500-bp band.
Patch clamp recordings
Conventional and perforated whole-cell patch clamp recordings were performed at room temperature in L-15 cell culture medium (Invitrogen) containing the following inorganic salts (in millimolar): NaCl (137), KCl (5.4), CaCl2 (1.26), MgCl2 (1.0), Na2HPO4 (1.0), KH2PO4 (0.44), MgSO4 (0.81). Organs of Corti cultured in the glass bottom Petri dishes were observed with an inverted microscope (Eclipse TE2000-U) using a ×100 1.3 NA 0.2WD oil immersion objective and differential interference contrast (Nikon, Tokyo, Japan). To get access to the basolateral wall of OHCs, the outermost supporting cells were removed by gentle suction with a ∼5-μm micropipette. Patch clamp pipettes were filled with intracellular solution containing (in millimolars): CsCl (140), MgCl2 (2.5), Na2ATP (2.5), EGTA (1.0), HEPES (5). Recordings were performed with AxoPatch 200B amplifier (Molecular Devices, Sunnyvale, CA). The pipette resistance was typically 2–5 MΩ when measured in the bath. For perforated patch clamp recordings, 50 μg/ml of gramicidin (Sigma-Aldrich, St. Louis, MO) was added to the intrapipette solution. Gigaohm seal was established at the basolateral surface of an OHC. Whole-cell recording configuration was obtained using the “zap” feature of the AxoPatch amplifier in the conventional recordings or waiting for 1–5 min under continuous “membrane test” control in perforated recordings. Series resistance was approximately 5–50 MΩ in conventional and 20–90 MΩ in perforated recordings. OHC responses were recorded at the holding potential of −70 mV. To obtain current–voltage relationships (I–V curves), voltage ramps from −120 to 100 mV were periodically applied. All I–V curves were corrected for the voltage drop across series resistance.
Drug delivery
Mustard oil (allyl isothiocyanate, AITC), cinnamaldehyde (CA), and icilin were obtained from Sigma-Aldrich. To prepare stock solutions, 4.9 μl of AITC was added to 250 μl of anhydrous ethanol, 6.3 μl of CA was added to 250 μl of 60% ethanol, and 10 mg of Icilin was added to 642.5 μl of anhydrous dimethyl sulfoxide (DMSO; Sigma-Aldrich). Prepared stock solution of 4HNE in methanol (10 mg/ml) was purchased from Cayman Chemical Company (Ann Arbor, MI). Stock solutions were diluted with extracellular medium to final working concentrations of 10–20 μM (AITC), 100–200 μM (CA), 100 μM (icilin), and 50 μM (4HNE). The final concentrations of the solvent were less than 0.05% (AITC), 0.1% (CA), 0.2% (icilin), and 0.08% (4HNE). All these TRPA1 agonists were applied to OHCs through a puff pipette with a tip diameter of ∼1 μm by applying to the pipette a pressure of ∼1.5 psi using PDES-02T pneumatic drug injector (NPI Electronics, Tamm, Germany). Special care was taken to make the flow as gentle as possible and direct it behind the stereocilia to avoid activation of mechanotransduction channels (Fig. 1A). Under these experimental conditions, control applications of the bath solution without drugs did not deflect stereocilia and did not generate any noticeable whole-cell current responses (data not shown).
Mouse OHCs possess functional TRPA1 channels. A Bright-field image of the organ of Corti showing the experimental arrangement. Conventional or gramicidin-based perforated whole-cell patch clamp recordings were established at the basolateral surface of OHCs (pipette on the right). Various TRPA1 agonists were delivered through a glass pipette at the top. Scale bar, 10 μm. B, C Representative whole-cell current responses to the application of TRPA1 agonists in OHCs of wild-type (Trpa1 +/+) (B) and in the Trpa1 knockout (Trpa1 −/−) (C) mice. Concentrations of TRPA1 agonists were 10 μM (allyl isothiocyanate, AITC), 100 μM (icilin), 100 μM (cinnamaldehyde, CA), and 50 μM (4-hydroxynonenal, 4HNE). Horizontal bars indicate the timing of drug application. D–F Insets, Representative current–voltage (I–V) relationships in an OHC before (black) and during (gray) application of 100 μM of CA (D, F) or 50 μM of 4HNE (E) in wild-type (D, E) and Trpa1 knockout (F) animals. Holding potential was gradually decreased from a normal resting value of −70 to −120 mV in 0.5 s. Then, I–V data were collected during a linear sweep of the potential from −120 to +100 mV in 2–3 s. Main graphs, Average current–voltage relationships of the TRPA1-mediated responses (gray open symbols) derived by subtracting I–V curves before and during application of TRPA1 agonist. The control “washout” graphs (black solid symbols) were obtained by subtracting I–V curves before and 5–10 min after the application of the agonist. The data are shown as mean ± SE. Number of cells/animals: 8/5 (D), 8/4 (E), and 5/3 (F). Age of the specimens: P2-4 plus 1–5 days in vitro. All cells were located approximately in the middle of the cochlea.
Heterologous expression of TRPA1 channels
COS-7 or HEK293 cells (ATCC, Manassas, VA) were plated on 50-mm glass bottom dishes (Willco Wells). Cells were maintained in DMEM cell culture medium (Invitrogen) supplemented with 7% FBS (Atlanta Biologicals, Lawrenceville, GA) and 10 μg/ml ampicillin (Invitrogen) at 37°C and 5% CO2. After 20–24 h, when cell layer confluency reaches 70–80%, the medium was changed to serum-free Opti-Mem (Invitrogen). Using Lipofectamine 2000 (Invitrogen), the cells were transfected with a bicistronic construct expressing both AcGFP1 and FLAG-tagged mouse TRPA1 in the same cell. This expression construct was generated by cloning a full-length mouse cDNA Trpa1 insert (nucleotide accession no. NM_177781) into the pIRES2-AcGFP1 vector (Clontech Laboratories Inc., Mountain View, CA). Patch clamp recordings of TRPA1-mediated current responses and gentamicin–Texas Red conjugate (GTTR) uptake experiments were performed at 20–28 h after transfection.
Gentamicin–Texas Red conjugate
Gentamicin (Sigma-Aldrich) was conjugated with Texas Red succinimidyl esters (Molecular Probes, OR) and purified as previously described (Myrdal et al. 2005). To prepare a stock solution, dried GTTR conjugate was reconstituted to 1 mg/ml in DMSO. To prepare a working solution, the stock solution was further diluted to 2 μg/ml either with standard Hank’s balanced salt solution (HBSS) containing 1.26 mM of Ca2+ and 0.9 mM of Mg2+ (catalog no. 14025, Invitrogen) or with Ca2+-free HBSS (catalog no. 14175, Invitrogen) supplemented with 0.5 mM of MgCl2.
TRPA1-mediated uptake of GTTR in heterologous cells
The amount of GTTR that enters the cell via TRPA1 channels was assessed by comparing cell fluorescence after brief incubation with GTTR for 20 s at room temperature with or without TRPA1 agonists in the bath. We chose only the cells that were firmly adhered to the glass and had normal epithelial-like morphology. Floating cells or the cells that appeared rounded were not included in analysis. Cells transfected with the vector encoding only GFP were used as a control. After incubation, the cells were rinsed with standard (Ca2+-containing) HBSS to remove any extracellular GTTR. GTTR that entered the cell was trapped there by the negative intracellular potential and therefore cannot be washed out. Within 5–15 min after incubation with TRPA1 agonist, cells were observed with an Axiovert 200 M microscope equipped with a Plan-Apochromat ×100 1.4 NA oil immersion objective and the LSM 5 Live laser confocal scanning module (Carl Zeiss, Jena, Germany). GTTR was excited at 532 nm and GFP was excited at 488 nm. Interference emission filters of 560–675 nm and 500- to 525-nm bandwidths were used to collect GTTR and GFP fluorescence, correspondingly. The pinhole was adjusted to obtain optical sections of ∼2-μm thickness. Typically, this thickness was enough to cover the height of generally flat COS-7 cells adhered to the bottom of the dish, with the exception of the nucleus region. Images were acquired using LSM software (Carl Zeiss).
TRPA1-mediated loading of GTTR in the organ of Corti explants
To avoid entry of GTTR into hair cells via mechanotransduction channels, organ of Corti explants were first pre-incubated for 10 min in Ca2+-free HBSS supplemented with 10 mM 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid (BAPTA) and 0.5 mM MgCl2. This treatment disrupts mechanotransduction machinery in both non-mammalian (Assad et al. 1991; Zhao et al. 1996) and mammalian (Beurg et al. 2006; Stepanyan and Frolenkov 2009) hair cells. It is also likely to break the tight junction barrier between the apical and basolateral compartments of the polarized epithelium (Farshori and Kachar 1999). The specimens were then incubated in GTTR (Myrdal et al. 2005) for 20 s at room temperature with or without TRPA1 agonists in the bath. After incubations, all samples were carefully rinsed with Ca2+-free HBSS supplemented with 0.5 mM MgCl2 and 50 μM CaCl2. To assess the effectiveness of BAPTA pretreatment, some control samples untreated with BAPTA were also incubated in GTTR. Special care was taken to use the identical protocol in all samples to be compared (as in Fig. 3), and all procedures with these specimens were done in parallel. Within 5–15 min after incubation, the organs of Corti were observed with a LSM 5 Live laser confocal microscope (Carl Zeiss), as described above. Stacks of optical sections of ∼2-μm thickness were acquired and the images at the focal plane across the middle of OHC bodies were chosen for illustrations.
FM1-43 loading
FM1-43 (Invitrogen) was dissolved in DMSO to obtain a stock solution with a concentration of 1 mM. Immediately before the experiment, the stock solution was diluted to 5 μM in Ca2+-free HBSS. Similar to GTTR uptake experiments, we compared the amount of FM1-43 loaded in OHCs with or without TRPA1 agonists. To disrupt the mechanotransduction machinery, we pre-incubated the specimens in Ca2+-free HBSS supplemented with 10 mM BAPTA and 0.5 mM MgCl2 for 5 min. Then, all specimens were briefly incubated in FM1-43 for 30 s and then carefully rinsed in standard HBSS. All incubations were performed at room temperature. Explants were observed using an upright Olympus BX51W1 microscope (Olympus, Center Valley, PA) with a LUMplan FL ×60 1.0 NA water immersion objective (Olympus). The FM1-43 fluorescence was excited at ∼488 nm and observed at ∼525 nm. Images were acquired with an Evolve 512 camera (Photometrics, Tucson, AZ) and MetaMorph software (Molecular Devices).
Results
Cochlear outer hair cells possess functional TRPA1 channels
Because Trpa1 knockout mice exhibit apparently normal hearing and hair cell mechanotransduction, the function of TRPA1 channels in mammalian hair cells is unclear (Bautista et al. 2006; Kwan et al. 2006). Unfortunately, the exact subcellular localization of these channels cannot be determined using immunolabeling techniques because all TRPA1 antibodies tested by us and other groups have failed the specificity tests in the inner ear tissues of Trpa1 knockout animals (our unpublished data and personal communication from Dr. David P. Corey). Therefore, we explored whether mammalian OHCs express functional TRPA1 channels using electrophysiological techniques. We established whole-cell patch clamp recordings from OHCs in the young postnatal cultured organ of Corti explants and investigated the responses of these cells to puff application of TRPA1 agonists (Fig. 1A). We used several pungent compounds to activate TRPA1 channels including mustard oil (also known as AITC), icilin, CA, and an endogenously generated agonist, 4HNE. All these compounds activate TRPA1 channels (Story et al. 2003; Bandell et al. 2004; Jordt et al. 2004; Bautista et al. 2005; Nagata et al. 2005; Macpherson et al. 2007b; Trevisani et al. 2007), some of them (CA, AITC) being highly selective (Bandell et al. 2004; Jordt et al. 2004). In OHCs of wild-type mice, all TRPA1 agonists produced prominent inward current responses (Fig. 1B), while TRPV1 agonist capsaicin (a negative control) did not evoke any responses in OHCs (data not shown). Voltage dependence of TRPA1 responses in the wild-type OHCs often showed some nonlinearities at high transmembrane potentials (Fig. 1D, E) that are typical for heterologously expressed TRPA1 channels in the near-physiological ionic environment (Story et al. 2003; Kim and Cavanaugh 2007; Macpherson et al. 2007b), but not in symmetrical solutions or two-cation patch clamp recordings (Story et al. 2003; Karashima et al. 2010). The reversal potential of the current responses evoked by TRPA1 agonists in wild-type OHCs was close to zero, as expected for non-selective cation conductance (Fig. 1D, E).
In conventional whole-cell recordings, TRPA1 responses were observed in approximately 65% of the cells (total number of cells tested, n = 43) in the first 2 min after establishing whole-cell conditions and were greatly reduced thereafter. In perforated patch recordings, TRPA1 responses were evoked in 75% of the cells (n = 8) and even after 10–20 min of whole-cell recording. The advantage of perforated patch recording for TRPA1 responses is not surprising because activation of a relatively low number of TRPA1 channels in an OHC is expected to be “self-amplified” by a rise of intracellular Ca2+ concentration in the vicinity of the channels (Doerner et al. 2007; Zurborg et al. 2007). This “self-amplification” may be affected by exogenous Ca2+ chelators that diffuse from the patch pipette into the cell during conventional whole-cell recordings.
Next, we used the same patch clamp techniques to test TRPA1 reponses in OHCs of young postnatal Trpa1 knockout mice (Trpa1 −/−). None of the OHCs tested (n = 15) exhibited a significant (>3 pA) inward current response to the application of TRPA1 agonists either in conventional or in perforated recordings (Fig. 1C, F). Thus, we concluded that cochlear OHCs possess functional TRPA1 channels.
Exogenous TRPA1 channels are permeable to GTTR
To determine whether TRPA1 channels can provide a pathway for the entry of gentamicin into the cell, we transfected heterologous cells with a bicistronic expression construct that translates individually both sequence-verified mouse TRPA1 and a GFP reporter in the same cell. Approximately 20 h after transfection, we briefly incubated cells for 30 s in the GTTR-containing bath with or without the TRPA1 agonist, CA. This short incubation time, at room temperature, was chosen to diminish the contribution of GTTR uptake by endocytosis (Gale et al. 2001). After incubation and washout, the intensity of intracellular GTTR fluorescence was determined using confocal imaging. GFP-positive transfected cells stimulated by CA did load GTTR (Fig. 2A, left). This uptake was proportional to the intensity of the GFP reporter, which is presumably proportional to the efficiency of transfection (Fig. 2B). We did not observe significant GTTR fluorescence in Trpa1-transfected cells that were not stimulated by CA (Fig. 2A, middle). We also did not observe any significant GTTR fluorescence in the cells transfected with a control vector expressing GFP but no TRPA1, even after an identical stimulation with CA (Fig. 2A, right, and B).
Heterologously expressed mammalian TRPA1 channels are permeable to GTTR. A Laser confocal imaging of GTTR uptake in cultured COS-7 cells transfected with a bicistronic expression FLAG–Trpa1–IRES–GFP construct encoding separately mouse TRPA1 and GFP in the same cell (left and middle panels). Control cells were transfected with an expression vector encoding only GFP (right panels). Left panels show TRPA1-expressing cells that were stimulated with the TRPA1 agonist, CA. Middle panels show TRPA1-expressing cells that were not stimulated with CA. Right panels show cells that do not express TRPA1 but were stimulated with CA (control mock-transfected cells). All cells were pre-incubated with GTTR at room temperature for 30 s and then washed two to three times. CA (200 μM) was added to the incubating medium in the experiments on the left and right panels. From top to bottom: GTTR fluorescence (red), GFP reporter fluorescence (green), and the merged image of the red and green channels. B Scatter plot of GTTR fluorescence intensity versus GFP reporter fluorescence in the TRPA1-expressing (closed black symbols; correlation coefficient r = 0.8799, p < 0.0001) and control (open gray symbols; correlation coefficient r = 0.1418, p = 0.52) cells. All cells were stimulated with 200 μM of CA. Dashed lines show least square linear fit through the corresponding data. C Typical whole-current responses to the application of 20 μM of TRPA1 agonist AITC in Trpa1-transfected HEK293 cells (top), non-transfected (non-fluorescent) cells in the same dish (middle), and mock-transfected cells (the same vector translating only GFP, bottom). Horizontal gray bars indicate timing of application of TRPA1 agonist. All recordings were made 20 h following transfection. Right image shows typical microscopic appearance of transfected (green) and non-transfected (black and white) HEK293 cells. Bright-field image was superimposed with GFP fluorescence. Scale bar, 40 μm.
We also examined the whole-cell current responses in Trpa1-transfected heterologous cells. In correspondence with numerous previous studies (Story et al. 2003; Bandell et al. 2004; Jordt et al. 2004; Bautista et al. 2005; Nagata et al. 2005; Macpherson et al. 2007a), we observed robust inward current responses to the application of the most commonly used TRPA1 agonist, AITC (Fig. 2C), confirming the ability of our construct to express functional TRPA1 channels. Similar to TRPA1 responses in OHCs, TRPA1-mediated currents in heterologous cells reversed at the holding potential close to zero, which is consistent with non-selective cation conductance (data not shown). Non-transfected cells from the same dish, as well as the cell transfected with a vector encoding only GFP (mock-transfected), did not exhibit any responses to AITC (Fig. 2C).
Endogenous TRPA1 channels in OHCs allow permeation of GTTR and other small organic cations
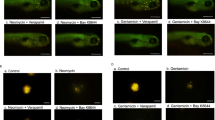
To investigate the uptake of gentamicin in OHCs, we incubated cultured organ of Corti explants of young postnatal mice in GTTR for 20 s at room temperature. This short duration of drug exposure at room temperature was chosen to diminish GTTR uptake by endocytosis. When organ of Corti explants were incubated with GTTR, a strong fluorescent signal was observed in the OHCs of both wild-type and Trpa1 knockout mice (Fig. 3A, first row). There was also a faint but distinct signal in the inner hair cells of both wild-type and Trpa1 knockout mice (Fig. 3A, first row). The accumulation of GTTR was largely inhibited after disrupting hair cell mechanotransduction by pretreatment of organ of Corti explants with Ca2+-free extracellular solution supplemented with BAPTA (Fig. 3A, second row). We concluded that GTTR is able to permeate the mechanotransduction channels in OHCs even though the diameter of the GTTR molecule is expected to be larger than the diameter of the unconjugated gentamicin. This finding is consistent with a recent conference report from Anthony Ricci’s group (Luk et al. 2010).
Endogenous TRPA1 channels in the organ of Corti explants are permeable to GTTR. A Confocal images of GTTR fluorescence in live organ of Corti explants of wild-type (left panels) and Trpa1 knockout mice (right panels). All specimens were incubated with GTTR for 20 s at room temperature. From top to bottom: (1) control specimens untreated with BAPTA or TRPA1 agonists; (2) specimens pretreated with Ca2+-free media containing 10 mM BAPTA for 10 min; (3) explants pretreated with BAPTA as above and then incubated with 200 μM of CA for 20 s (together with GTTR); and (4) specimens treated with BAPTA as above and then incubated with 50 μM of endogenous TRPA1 agonist 4HNE for 20 s (together with GTTR). B Average intensities of GTTR fluorescence at the focal plane across the middle of an OHC cylindrical body in wild-type (white bars) and Trpa1 knockout mice (gray bars). TRPA1 agonists CA and 4HNE significantly increase GTTR uptake in wild-type but not in Trpa1 knockout OHCs (*p < 0.001; Student’s t test for independent variables, error bars = SEM). OHCs outer hair cells, IHCs inner hair cells, HCs Hensen’s cells. Number of analyzed OHCs (number of explants): 81 (4) (control, Trpa1 +/+); 71 (2) (control, Trpa1 −/−); 138 (4) (BAPTA, Trpa1 +/+); 75 (2) (BAPTA, Trpa1 −/−); 56 (2) (BAPTA-CA, Trpa1 +/+); 27 (2) (BAPTA-CA, Trpa1 −/−); 46 (2) (BAPTA-4HNE, Trpa1 +/+); 51 (2) (BAPTA-4HNE, Trpa1 −/−). All specimens were processed in parallel using the identical protocol. All images were acquired from the middle turn of the cochlea using identical exposure times, pinhole values, detector gain, laser attenuation, and other acquisition settings. Scale bars, 20 μm. Age of the specimens: P3-4 plus 2 days in vitro.
Next, we explored whether the activation of TRPA1 channels results in GTTR loading in mouse OHCs. After pretreatment with BAPTA, brief incubation of organ of Corti explants with TRPA1 agonists for 20 s resulted in a significant increase of GTTR fluorescence in wild-type but not in Trpa1 knockout OHCs (Fig. 3A, third and fourth rows). Extracellular application of both TRPA1 agonists, exogenous cinnamaldehyde at 100 μM and the endogenous agonist 4HNE at 50 μM, produced an increase of GTTR uptake, albeit with different efficacies. It is interesting to note that TRPA1-mediated uptake of GTTR was not limited to OHCs, but was also observed in Hensen’s and other supporting cells (Fig. 3A, third row, left panel). This observation is consistent with earlier indications of a broad expression of TRPA1 within the cochlea (Corey et al. 2004). Quantification of the GTTR signal in organ of Corti explants showed a ∼5.7-fold decrease in GTTR fluorescence in OHCs after the disruption of mechanotransduction channels. Subsequent activation of TRPA1 channels resulted in a ∼6.1-fold increase of GTTR fluorescence in wild-type but not in Trpa1 knockout OHCs (Fig. 3B).
If the mechanism of gentamicin permeation through TRPA1 channels is similar to other non-selective cation channels, then the TRPA1 pore should allow permeation of other small organic cations (Farris et al. 2004; Marcotti et al. 2005). We explored whether TRPA1 channels in the OHCs are permeable to another small organic cation, FM1-43. FM1-43 is a small styryl dye that can permeate through the mechanotransduction channels of mammalian hair cells and through other non-selective cation channels (Gale et al. 2001; Meyers et al. 2003). As expected, incubation of control (untreated with BAPTA) OHCs with FM1-43 resulted in an equally strong labeling in both wild-type and Trpa1 knockout mice (Fig. 4A, first row). Similar incubation with FM1-43 but in the presence of TRPA1 agonist, CA, resulted in a larger uptake of the dye in wild-type OHCs than in Trpa1 knockout OHCs (Fig. 4A, second row, and D). We disrupted the entry of FM1-43 into the OHCs through mechanotransduction channels by pre-incubating organ of Corti explants in Ca2+-free extracellular medium supplemented with BAPTA. As expected, BAPTA treatment prevented entry of the dye into the OHCs in explants from both wild-type and Trpa1 knockout mice (Fig. 4A, third row). However, activation of TRPA1 channels in BAPTA-treated specimens resulted in the accumulation of FM1-43 in the OHCs of wild-type but not Trpa1 knockout mice (Fig. 4A, fourth row, and B, D). Similar FM1-43 uptake was obtained in wild-type but not Trpa1 knockout OHCs stimulated by another TRPA1 agonist, AITC (data not shown). To exclude the possibility that FM1-43 uptake in the OHCs is due to a secondary activation of ATP-gated P2X channels (Meyers et al. 2003; Crumling et al. 2009), we repeated this experiment in the presence of pyridoxalphosphate-6-azophenyl-29,49-disulfonic acid (PPADS), a non-selective blocker of ATP receptors. PPADS did not inhibit the uptake of FM1-43 evoked by TRPA1-agonists (Fig. 4C). We concluded that endogenous TRPA1 channels in the organ of Corti are permeable to GTTR, FM1-43, and probably other small organic cations.
Endogenous TRPA1 channels in the mouse organ of Corti are permeable to FM1-43. A FM1-43 uptake in the cultured organ of Corti explants of wild-type (left panels) and Trpa1 knockout (right panels) mice following 30 s of incubation with 5 μM of the dye at room temperature. From top to bottom: (1) control organ of Corti explants not treated with BAPTA or TRPA1 agonists; (2) explants not treated with BAPTA but incubated with 200 μM of TRPA1 agonist, CA together with FM1-43; (3) specimens treated for 15 min in Ca2+-free media containing 10 mM BAPTA before incubating in FM1-43; and (4) explants pretreated with BAPTA and incubated with 200 μM of CA together with FM1-43. B Orthogonal view generated from confocal images of the organ of Corti explant from a wild-type mouse pretreated with BAPTA and incubated with 200 μM CA together with FM1-43. C FM1-43 uptake in an explant from a wild-type mouse pretreated with BAPTA and incubated with CA (200 μM) supplemented with 50 μM of a non-selective P2 purinergic antagonist, PPADS. D Average intensities of FM1-43 fluorescence in OHCs of wild-type (white bars) and Trpa1 knockout mice (gray bars). TRPA1 agonists CA significantly increase GTTR uptake in wild-type but not in Trpa1 knockout OHCs (*p < 0.05; **p < 0.01; Student’s t test for independent variables, error bars = SEM). Number of analyzed OHCs (number of explants): 47 (2) (control, Trpa1 +/+); 90 (2) (control, Trpa1 −/−); 74 (2) (control-CA, Trpa1 +/+); 63 (2) (control-CA, Trpa1 −/−); 47 (2) (BAPTA, Trpa1 +/+); 45 (2) (BAPTA, Trpa1 −/−); 63 (2) (BAPTA-CA, Trpa1 +/+); 27 (2) (BAPTA-CA, Trpa1 −/−). All specimens were processed and imaged using identical protocols and imaging parameters. All cells were located approximately in the middle of the cochlea. Age of the specimens: P3-4 plus 2 days in vitro. Scale bars, 20 μm. OHCs outer hair cells, IHCs inner hair cells.
Discussion
This study demonstrates that mammalian cochlear OHCs possess functional TRPA1 channels and that these channels are permeable to the aminoglycoside antibiotic, gentamicin, and another small organic cation, FM1-43.
Expression of functional TRPA1 channels in OHCs
First evidence for the expression of TRPA1 channels in mammalian cochlear hair cells were provided by Corey et al. (2004) who proposed that this protein might function as the mechanotransduction channel. However, this hypothesis was subsequently challenged by apparently normal hearing function and hair cell mechanotransduction in Trpa1 knockout mice (Bautista et al. 2006; Kwan et al. 2006). Yet, a PLAP reporter driven by the Trpa1 promoter in Trpa1 knockout animals (Kwan et al. 2006) shows widespread expression in the organ of Corti, including hair cells, Hensen’s cells, and other supporting cells (Kwan K.Y., personal communication).
Using patch clamp and live cell imaging techniques, we demonstrate in this study that murine OHCs possess functional TRPA1 channels. The exact function of TRPA1 channels in the OHCs and other cells of the organ of Corti remains to be identified. However, a predominant expression of TRPA1 channels in the nociceptive subset of somatic afferent sensory neurons (Story et al. 2003; Nagata et al. 2005; Bautista et al. 2006; Kwan et al. 2006), the activation of these channels by exogenous and endogenous noxious compounds (Bandell et al. 2004; Jordt et al. 2004; Macpherson et al. 2005), and a possibility of the direct activation of TRPA1 through a covalent modification of cysteine residues (Hinman et al. 2006; Macpherson et al. 2007a) led to the commonly held hypothesis that TRPA1 channels may function as universal sensors for chemical damage (Macpherson et al. 2007a; Trevisani et al. 2007). It is interesting to note that a similar “damage sensor” function has been hypothesized for ATP receptors ubiquitously expressed in the cochlea (Gale et al. 2004). Our data are consistent with the idea that TRPA1 channels have a widespread distribution in the organ of Corti and may work together with ATP receptors to detect endogenous byproducts of damage occurring in the cochlea.
Permeability of TRPA1 channels to gentamicin and other small organic cations
Irrespective of the exact function of TRPA1 in the cochlea, these channels may provide a pathway for the entry of aminoglycosides into OHCs, if they are activated during systemic administration of antibiotics. Indeed, TRPA1 is a large non-selective cation channel with pharmacological properties that are similar to the properties of hair cell mechanotransduction channels (Nagata et al. 2005). Notably, the Hill coefficient and IC50 for gentamicin binding are indistinguishable between the hair cell mechanotransduction channels and TRPA1 channels (Nagata et al. 2005). Hair cell mechanotransduction channels have a pore of about 1.25 nm in diameter (Farris et al. 2004). These channels allow permeation by small organic cations (Corey and Hudspeth 1979; Ohmori 1985; Marcotti et al. 2005) such as the aminoglycoside antibiotic dihydrostreptomycin (elongated molecule with end-on diameter 0.8 nm), tetraethylammonium ion with a diameter of 0.82 nm, and FM1-43, another elongated molecule with a “bulky” tetraethylammonium head group and a diameter of 1.06 nm (Gale et al. 2001).
Permeation of FM1-43 through exogenously expressed TRPA1 channels has been also demonstrated (Karashima et al. 2010). Moreover, TRPA1 channels are permeable to other organic cations, such as N-methyl-d-glucamine and carbocyanine nucleic acid stain YO-PRO®-1 (Banke et al. 2010). Therefore, it is not surprising that TRPA1 channels with an estimated pore diameter between 1.1 and 1.4 nm (Karashima et al. 2010) are permeable to gentamicin molecules with a diameter of <1 nm (Kroese et al. 1989).
More puzzling is the fact that both hair cell mechanotransduction channels and TRPA1 channels are permeable to GTTR, which is expected to be significantly larger than an unconjugated gentamicin molecule. However, several characteristics other than molecular weight (physical dimensions, charge, hydrophobicity) impact the ability of a molecule to permeate through a particular channel (Steyger et al. 2003; Myrdal and Steyger 2005). Perhaps GTTR represents an elongated molecule with an overall size still within the limits imposed by the pore diameter of these channels. It has been reported that the permeability of the hair cell mechanotransduction channels is determined by the diameter, not by the length of the molecule (Farris et al. 2004). Regardless, we provide strong evidence for GTTR permeation through TRPA1 and mechanotransduction channels (Figs. 2 and 3). There is also an independent report on the ability of GTTR to penetrate through mechanotransduction channels in mammalian hair cells (Luk et al. 2010). Thus, we concluded that TRPA1 channels are permeable to both conjugated and unconjugated forms of gentamicin.
How could TRPA1 channels be activated in OHCs in vivo?
A number of different inflammatory agents can activate TRPA1 channels through direct or indirect mechanisms, including activation by endogenous agents produced during oxidative stress (Bandell et al. 2004; Bautista et al. 2006; Macpherson et al. 2007b; Andersson et al. 2008). According to our data, at least one of these agents, 4HNE, can activate TRPA1 channels in OHCs. Delayed generation of exactly this endogenous reagent has been observed in mammalian cochlea several days after damaging noise exposure (Yamashita et al. 2004). Therefore, our data suggest a novel pathway of aminoglycoside uptake into OHCs through TRPA1 channels that may be activated in a stressed cochlea.
References
Andersson DA, Gentry C, Moss S, Bevan S (2008) Transient receptor potential a1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 28:2485–2494
Assad JA, Shepherd GM, Corey DP (1991) Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7:985–994
Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41:849–857
Banke TG, Chaplan SR, Wickenden AD (2010) Dynamic changes in the TRPA1 selectivity filter lead to progressive but reversible pore dilation. Am J Physiol Cell Physiol 298:C1457–C1468
Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM (2005) Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA 102:12248–12252
Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124:1269–1282
Beurg M, Evans MG, Hackney CM, Fettiplace R (2006) A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J Neurosci 26:10992–11000
Blanchet C, Erostegui C, Sugasawa M, Dulon D (2000) Gentamicin blocks ACh-evoked K+ current in guinea-pig outer hair cells by impairing Ca2+ entry at the cholinergic receptor. J Physiol 525(Pt 3):641–654
Christensen AP, Corey DP (2007) TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci 8:510–521
Clapham DE (2003) TRP channels as cellular sensors. Nature 426:517–524
Corey DP (2003) New TRP channels in hearing and mechanosensation. Neuron 39:585–588
Corey DP, Hudspeth AJ (1979) Ionic basis of the receptor potential in a vertebrate hair cell. Nature 281:675–677
Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amalfitano A, Cheung EL, Derfler BH, Duggan A, Geleoc GS, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang DS (2004) TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432:723–730
Crumling MA, Tong M, Aschenbach KL, Liu LQ, Pipitone CM, Duncan RK (2009) P2X antagonists inhibit styryl dye entry into hair cells. Neuroscience 161:1144–1153
de Groot JC, Meeuwsen F, Ruizendaal WE, Veldman JE (1990) Ultrastructural localization of gentamicin in the cochlea. Hear Res 50:35–42
Doerner JF, Gisselmann G, Hatt H, Wetzel CH (2007) Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem 282:13180–13189
Farris HE, LeBlanc CL, Goswami J, Ricci AJ (2004) Probing the pore of the auditory hair cell mechanotransducer channel in turtle. J Physiol 558:769–792
Farshori P, Kachar B (1999) Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J Membr Biol 170:147–156
Forge A, Schacht J (2000) Aminoglycoside antibiotics. Audiol Neurootol 5:3–22
Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP (2001) FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci 21:7013–7025
Gale JE, Piazza V, Ciubotaru CD, Mammano F (2004) A mechanism for sensing noise damage in the inner ear. Curr Biol 14:526–529
Hashino E, Shero M (1995) Endocytosis of aminoglycoside antibiotics in sensory hair cells. Brain Res 704:135–140
Hiel H, Erre JP, Aurousseau C, Bouali R, Dulon D, Aran JM (1993) Gentamicin uptake by cochlear hair cells precedes hearing impairment during chronic treatment. Audiology 32:78–87
Hinman A, Chuang HH, Bautista DM, Julius D (2006) TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA 103:19564–19568
Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427:260–265
Karashima Y, Prenen J, Talavera K, Janssens A, Voets T, Nilius B (2010) Agonist-induced changes in Ca(2+) permeation through the nociceptor cation channel TRPA1. Biophys J 98:773–783
Kim D, Cavanaugh EJ (2007) Requirement of a soluble intracellular factor for activation of transient receptor potential A1 by pungent chemicals: role of inorganic polyphosphates. J Neurosci 27:6500–6509
Kimitsuki T, Ohmori H (1993) Dihydrostreptomycin modifies adaptation and blocks the mechano-electric transducer in chick cochlear hair cells. Brain Res 624:143–150
Kroese AB, Das A, Hudspeth AJ (1989) Blockage of the transduction channels of hair cells in the bullfrog’s sacculus by aminoglycoside antibiotics. Hear Res 37:203–217
Kros CJ, Rusch A, Richardson GP (1992) Mechano-electrical transducer currents in hair cells of the cultured neonatal mouse cochlea. Proc Biol Sci 249:185–193
Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP (2006) TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50:277–289
Lim DJ (1986) Effects of noise and ototoxic drugs at the cellular level in the cochlea: a review. Am J Otolaryngol 7:73–99
Luk L, Alharazneh A, Naeem T, Monfared A, Steyger P, Cheng A, Ricci A (2010) Aminoglycosides rapidly and selectively enter hair cells, largely via mechanotransducer channels. 33rd Annual MidWinter Research Meeting of the Association for Research in Otoraryngology, Anaheim, CA
Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A (2005) The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol 15:929–934
Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A (2007a) Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445:541–545
Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A (2007b) An ion channel essential for sensing chemical damage. J Neurosci 27:11412–11415
Marcotti W, van Netten SM, Kros CJ (2005) The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol 567:505–521
Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP (2003) Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci 23:4054–4065
Myrdal SE, Steyger PS (2005) TRPV1 regulators mediate gentamicin penetration of cultured kidney cells. Hear Res 204:170–182
Myrdal SE, Johnson KC, Steyger PS (2005) Cytoplasmic and intra-nuclear binding of gentamicin does not require endocytosis. Hear Res 204:156–169
Nagata K, Duggan A, Kumar G, Garcia-Anoveros J (2005) Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25:4052–4061
Ohmori H (1985) Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol 359:189–217
Ricci A (2002) Differences in mechano-transducer channel kinetics underlie tonotopic distribution of fast adaptation in auditory hair cells. J Neurophysiol 87:1738–1748
Rizzi MD, Hirose K (2007) Aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg 15:352–357
Russell IJ, Richardson GP (1987) The morphology and physiology of hair cells in organotypic cultures of the mouse cochlea. Hear Res 31:9–24
Russell IJ, Richardson GP, Cody AR (1986) Mechanosensitivity of mammalian auditory hair cells in vitro. Nature 321:517–519
Stepanyan R, Frolenkov GI (2009) Fast adaptation and Ca2+ sensitivity of the mechanotransducer require myosin-XVa in inner but not outer cochlear hair cells. J Neurosci 29:4023–4034
Steyger PS, Peters SL, Rehling J, Hordichok A, Dai CF (2003) Uptake of gentamicin by bullfrog saccular hair cells in vitro. J Assoc Res Otolaryngol 4:565–578
Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112:819–829
Strassmaier M, Gillespie PG (2003) Fast adaptation in the mammalian cochlea: a conserved mechanism for cochlear amplification. Nat Neurosci 6:790–791
Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P (2007) 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA 104:13519–13524
Yamashita D, Jiang HY, Schacht J, Miller JM (2004) Delayed production of free radicals following noise exposure. Brain Res 1019:201–209
Zhao Y, Yamoah EN, Gillespie PG (1996) Regeneration of broken tip links and restoration of mechanical transduction in hair cells. Proc Natl Acad Sci USA 93:15469–15474
Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA (2007) Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci 10:277–279
Acknowledgments
We thank Drs. Kelvin Y. Kwan and David P. Corey for providing us with Trpa1 knockout mice and Mrs. Stephanie Edelmann for maintaining mouse colony and genotyping the mice. We also thank Drs. David P. Corey, Bradley K. Taylor, Nuria Gavara, and Lisa L. Cunningham for critical reading of our manuscript. This work was supported by National Organization for Hearing Research Foundation (to RS) and NIDCD/NIH (DC009434 to GIF), as well as NIDCD Intramural funds (DC00048 to TBF) and NIDCD/NIH (DC004555 to PSS).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Stepanyan, R.S., Indzhykulian, A.A., Vélez-Ortega, A.C. et al. TRPA1-Mediated Accumulation of Aminoglycosides in Mouse Cochlear Outer Hair Cells. JARO 12, 729–740 (2011). https://doi.org/10.1007/s10162-011-0288-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-011-0288-x