Abstract
Background
Heavy proteinuria at diagnostic renal biopsy has been reported as an independent risk factor for deteriorating renal function in benign nephrosclerosis (BNS). However, studies investigating the relationship between the amount of proteinuria during follow-up and long-term renal prognosis in BNS are limited. This study aimed to assess the relationship between time-averaged proteinuria (TAP) and renal prognosis in BNS.
Methods
The study participants included 98 patients with biopsy-proven BNS (average age 52 ± 13 years, estimated glomerular filtration rate (eGFR) 53 ± 25 ml/min/1.73 m2, urine protein excretion at baseline 1.34 ± 1.30 g/gCr) from the Jikei University Hospital. Multivariate analysis was used to investigate the effects of TAP and other clinicopathological findings on the risk for renal outcome in biopsy-proven BNS (a 30% decline in eGFR from baseline or end-stage renal disease). Proteinuria was measured every 6 months and the mean value was used as an indicator of TAP.
Results
The average observation period was 56 ± 43 months. In the unadjusted model, higher levels of TAP and urinary protein at baseline, glomerulosclerosis, and tubulointerstitial damage were associated with renal prognosis. The adjusted model demonstrated a significant association between TAP and renal outcomes (hazard ratio 5.45, 95% confidence interval 3.02–10.7), which was independent of higher baseline proteinuria, glomerulosclerosis, and tubulointerstitial damage.
Conclusions
TAP is an independent risk factor for renal prognosis in patients with BNS, indicating the significance of urinary protein excretion during follow-up for the progression of BNS. Clinicians should understand the importance of follow-up evaluation for proteinuria in patients with BNS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Chronic kidney disease (CKD) represents an increasing health burden and is recognized as an important problem for both the individual and the society. First, CKD is not only a risk factor for end-stage kidney disease (ESKD) but also for cardiovascular disease (CVD), which is the main cause of death worldwide [1,2,3]. Second, the worldwide medical expenses associated with hemodialysis due to ESKD are expected to increase to a thousand billion US dollars within the next 10 years [4]. For these reasons, establishing effective CKD preventative measures is important; in fact, this is one of the most important issues in public and national health.
The number of patients with benign nephrosclerosis (BNS), which is one of the major causes of CKD, has been increasing worldwide. BNS is a common cause of ESKD, accounting for 28.4% and 12.3% of the ESKD cases [5, 6] in US and Japan, respectively. Furthermore, in an aging society, the increase in number of patients with BNS is expected to accelerate.
Accumulating evidence shows that heavy proteinuria at the time of diagnostic renal biopsy is an independent risk factor for deteriorating renal function in BNS [7]. In a previous study, on a case of idiopathic glomerulonephritis, such as immunoglobulin (Ig) A nephropathy (IgAN), an association was found between proteinuria during follow-up and renal prognosis rather than proteinuria at baseline [8, 9]. However, studies investigating the relationship between the amount of proteinuria during follow-up and long-term renal prognosis in BNS are lacking. Therefore, this study aimed to assess the relationship between time-averaged proteinuria (TAP) and renal prognosis in BNS.
Methods
Patient population
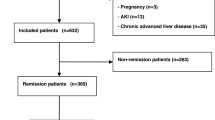
A total of 144 patients with biopsy-proven BNS who underwent native renal biopsies at the Jikei University Hospital and the Jikei Kashiwa University Hospital between April 2000 and December 2012 were enrolled. Patients who had a history of systemic disease, such as diabetes, malignant diseases, and collagen diseases (N = 29), patients who had malignant hypertension (N = 10), and those with a follow-up duration of < 12 months (N = 7) were excluded. Finally, 98 individuals were examined in this study (Fig. 1). Almost all patients had a history of hypertension.
Ethics issues
This study was conducted in accordance with the declaration of Helsinki. The study protocol was approved by the ethics review board of The Jikei University School of Medicine. All patients included in this study provided their written informed consent for performing renal biopsy and analyzing the data.
Renal function
Renal function was expressed by the estimated glomerular filtration rate (eGFR) using the following formula: eGFR (mL/min/1.73 m2) = (194 × creatinine (Cr) – 1.094) × (age – 0.287) (× 0.739, for women). This formula was proposed by the Japanese Society of Nephrology [10].
Clinical and laboratory information
Data on baseline demographics as well as clinical and laboratory data were reviewed prior to performing renal biopsy. Age, body mass index (BMI), blood pressure (BP), and medication history were obtained from each medical chart. Levels of hemoglobin, blood urea nitrogen, serum albumin, serum Cr, serum uric acid, total cholesterol, and hemoglobin A1c (HbA1c) (according to the National Glycohemoglobin Standardization Program) were measured using automated standardized methods at our hospital. Proteinuria was estimated by the spot urine protein–creatinine ratio.
The average observation period was 56 ± 43 months. Proteinuria was also measured every 6 months, and its mean value was used as an indicator of TAP [8, 9]. The mean value of proteinuria until 1 year was defined as proteinuria from baseline to 1 year.
BP during follow-up was also measured every 6 months, and the mean value was used for analyses.
Histopathological diagnosis
All renal tissue specimens were obtained via percutaneous needle biopsies. All specimens were examined using light microscopy and immunohistochemistry, including staining for IgG, IgA, IgM, C3, and C1q. The renal histopathological findings, including global glomerulosclerosis, segmental glomerulosclerosis, and interstitial fibrosis/tubular atrophy, were evaluated as previously described.
The pathological diagnosis of benign nephrosclerosis was made on the basis of typical histological features consistent with benign nephrosclerosis, including the presence of at least one of the following elements: intimal thickening of arteries, arteriolar hyalinosis, ischemic collapse or global sclerosis of glomeruli, or tubulointerstitial fibrosis [11, 12] Cases with immune deposits, except those with IgM as nonspecific deposit, were excluded.
Outcome
The renal outcome of this study was defined as either a decrease in the eGFR of 30% from baseline eGFR or from the start of the dialysis [13].
Statistical analysis
Continuous data were expressed as mean ± standard deviation or median with 25th and 75th percentiles, and categorical data were expressed as percentages. Renal survival and hazard ratio (HR) were estimated from the Cox regression model. Multiple analysis was used to calculate the HR for renal outcome after adjusting for sex, age, baseline eGFR, proteinuria (baseline), BMI, systolic BP (baseline), angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker (ACEi/ARB) use, global glomerulosclerosis rate, and interstitial fibrosis/tubular atrophy rate. Moreover, the association of each parameters and three different timings of proteinuria (baseline, from baseline to 1 year, and TAP) for renal outcome was investigated using multiple analysis.
In addition, the following analysis was performed for evaluating the sensitivity: to investigate the decline in kidney function, multiple regression analysis was used to calculate the standardized regression coefficients (β) and standard error (SE) by decline in eGFR after adjusting confounders.
Youden index was used to determine the best cut-off points for three different timings of proteinuria. Area under the curve (AUC) between 0.5 and 0.7 indicated “low accuracy”; between 0.7 and 0.8, “acceptable discrimination” or “moderate accuracy”; and between 0.8 and 0.9, “excellent discrimination” or “high accuracy” [14, 15].
The joint association of proteinuria (baseline) and TAP levels as categorical variables with the risk of renal events was also estimated as a secondary analysis. Patients with values less than each cut-off point served as the reference group in secondary analysis.
The following formula was used for analyzing the risk in patients with TAP ≥ 1.1 g/g for renal outcomes:
Bayes’ theorem,
where P(A) is the probability of event A; P(B), probability of event B; P(A/B), probability of event A under the condition that event B occurred; and P(B/A), probability of event B under the condition that event A occurred.
P value < 0.05 was considered statistically significant in the regression analyses. All statistical analyses were performed using EZR (version 1.33, Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, EZR is a modified version of R Commander designed to add statistical functions frequently used in biostatistics [16].
Results
The average observation period was 56 ± 43 months. 34 patients had a decline in eGFR over 30% and there were none who underwent dialysis during follow up. Table 1 shows the characteristics of participants at baseline and during follow-up. The mean age was 52.1 ± 12.7 years, and 73.5% of the patients were men. BP at baseline was 141.1 ± 14.7/84.9 ± 11.7 mmHg. The mean serum Cr was 1.45 ± 0.95 mg/dl, and eGFR was 52.7 ± 25.4 ml/min/1.73 m2. The proteinuria at baseline was 1.34 ± 1.3 g/gCr. ACEis/ARBs were used by 75.0% of patients. Histopathological parameters were as follows: global glomerulosclerosis rate was 33.2 ± 22.2%, and interstitial fibrosis/tubular atrophy rate was 28.3 ± 18.5%. The average values during follow-up were as follows: BP was 126.6 ± 7.7/77.9 ± 7.0, which was lower than BP at baseline. TAP was 1.07 ± 0.93 g/gCr, which was slightly lower than that at baseline.
Table 2 shows the unadjusted and adjusted HRs and 95% confidence intervals (CIs) for renal outcome according to each parameter. Sex, BMI, eGFR, global glomerulosclerosis rate, proteinuria at baseline, proteinuria from baseline to 1 year, and TAP were significantly associated with the renal outcome. With regard to age and interstitial fibrosis/tubular atrophy, a similar trend was observed, but it was not significant. TAP levels were positively associated with the risk of decline in renal function in both unadjusted (HR 2.59, 95% CI 1.83–3.62) and adjusted models (HR 3.46, 95% CI 2.06–5.83). The HR levels of TAP were higher than those of proteinuria at baseline or proteinuria from baseline to 1 year.
The results of multiple regression analysis were similar to those of the Cox regression model (Table 3). There was an association between TAP and decline in eGFR.
Table 4 shows the optimal cut-off point, AUC, and 95% CI of the three different timings of proteinuria for renal outcome. The optimal cut-off value of TAP for renal outcome was 1.1 g/gCr. The AUC was 0.79 (95% CI 0.69–0.88), which was considered “acceptable”. The optimal cut-off value of proteinuria from baseline to 1 year for renal outcome was 0.98 and the AUC was 0.74 (95% CI 0.64–0.84), which tended to be higher than the AUC of proteinuria at baseline. The longer the observation period for proteinuria, the higher was the HR and AUC for the renal outcome as per various models.
Using Bayes’ theorem, we revealed that (1) the probability for renal outcome was 0.82 under the condition of TAP ≥ 1.1 g/gCr, (2) the probability for renal outcome was 0.25 under the condition of TAP < 1.1 g/gCr, and (3) those with TAP ≥ 1.1 g/gCr have approximately 3.3 times higher risk of adverse renal outcomes than those with TAP < 1.1 g/Cr.
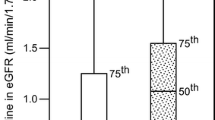
Figure 2 shows the joint association of baseline proteinuria and TAP levels with the risk for renal events. Those with a higher level of proteinuria/lower level of TAP had lower risk than those with a lower level of proteinuria/higher level of TAP. Cases with a high level of proteinuria at baseline and a low level of TAP had a lower risk than those with a low level of proteinuria at baseline and a high level of TAP.
Joint association of baseline proteinuria and time-averaged proteinuria levels with the risk for renal events. The joint association of baseline proteinuria (baseline) and TAP levels as categorical variables with the risk of renal events was also estimated as secondary analysis. Patients with proteinuria (baseline) < 1.4 g/gCr and TAP < 1.1 g/gCr served as the reference group in secondary analysis. Those with higher levels of proteinuria/lower levels of TAP had lower risk than those with lower levels of proteinuria/higher levels of TAP. Adjusted for age, sex, eGFR, proteinuria (baseline), body mass index, systolic BP (baseline), ACEI/ARB use, global glomerulosclerosis rate, and interstitial fibrosis/tubular atrophy rate. ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, BP blood pressure, CCB calcium channel blocker, CI confidence interval, Cr creatinine, eGFR estimated glomerular filtration rate, HR hazard ratio, TAP time-averaged proteinuria
Supplementary Table 1 shows the characteristics of study sample at baseline distributed by cut-off values of TAP. Patients with TAP ≥ 1.1 g/gCr had higher values of proteinuria at baseline and higher rates of global glomerulosclerosis than those with TAP < 1.1 g/gCr.
Discussion
This study assessed the relationship between TAP and renal prognosis in patients with biopsy-proven BNS. The present study suggests that TAP is an independent risk factor for renal prognosis in patients with BNS. More importantly, we revealed that the optimal cut-off value of TAP was 1.1 g/gCr, and those with TAP ≥ 1.1 g/gCr have approximately 3.3 times higher risk of adverse renal outcomes than those with TAP < 1.1 g/Cr.
The strengths of our study were as follows: (1) we found that TAP is an independent risk factor for renal prognosis in patients with BNS, and the cut-off value was estimated for preventing deterioration of renal function, and (2) our study was based on patients with biopsy-proven BNS.
Although proteinuria at baseline is a well-known risk factor for the progression of BNS, to our knowledge, no study has investigated the association between proteinuria during follow-up and renal failure progression in patients with BNS. Only a few studies investigating TAP in renal disease aside from BNS have been conducted, and the studies were limited to patients with IgA nephropathy [8, 9]. Our results are consistent with those of the previous studies, showing that TAP is a strong risk factor for renal function decrease, even after adjusting for some confounding factors. The primary target value of proteinuria differs for each nephropathy [17, 18]. In case of IgA nephropathy, a previous study indicated that TAP < 1.1 g/day was found to play an important role in limiting the loss of renal function in patients with IgA nephropathy [8]. Another retrospective study demonstrated the association between TAP and renal progression in glomerular diseases [18]. In the analysis with TAP, patients with IgA nephropathy had an increased risk for renal progression in proportion to the increasing level of proteinuria during treatment, even at a proteinuria level of 1.0–2.0 g/day, whereas renal progression was not observed in patients with membranous nephropathy (MGN) or focal segmental glomerulosclerosis (FSGS) with proteinuria level up to 3.0 g/g. Therefore, the goal for the value of TAP might be < 1.0 g/day in patients with IgAN and < 3.0 g/day in patients with MGN and FSGS [10]. However, the primary target value of TAP in case of BNS still needs to be fully elucidated. In the present study, we propose a cut-off value of follow-up proteinuria to prevent renal function decrease in patients with BNS. These findings suggest that reducing proteinuria to levels < 1.1 g/gCr over a long time is the primary goal of anti-proteinuria therapy and may seem to be the most important management option for the loss of renal function in patients with BNS.
Moreover, the diagnosis of BNS is usually a presumption because of the lack of histopathological confirmation, especially in patients with hypertension. Therefore, only a few studies have been conducted on patients with biopsy-proven BNS [12, 19, 20]. A retrospective study indicated that at baseline serum creatinine ≥ 200 µmol/l, systolic BP ≥ 160 mmHg and proteinuria ≥ 1 g/24 h were risk factors for end-stage renal disease in patients with biopsy-proven BNS [12]. Other studies showed that hyperuricemia was a predictive marker for progression of BNS [19]. However, these studies investigated only baseline parameters, but not parameters during follow-up.
The possibility that proteinuria may accelerate kidney disease progression to ESKD has received support from the results of several clinical studies [7, 21, 22]. Although proteinuria reflects the severity of glomerular damage, persistent proteinuria is the cause of exacerbating renal dysfunction. Protein–tubular cell interactions have inflammatory and fibrogenic consequences, which makes them the main feature of BNS [23, 24]. The possible hypotheses are as follows: this process occurs through multiple pathways, including induction of tubular chemokine expression and complement activation that leads to inflammatory cell infiltration in the interstitium and sustained fibrogenesis. Excessive protein loading on the proximal tubule cells is believed to be an inflammatory cell activity such as the macrophage; as a result, accompanying interstitial qualitative impairments have been reported [25]. In the present study, patients with high values of TAP had higher rates of global glomerulosclerosis at baseline. These may be due to the interactions between broken-down tissue and a high value of TAP. As our study showed, longer the observation period for proteinuria, higher was the risk for worse renal outcome. Thus, we suggest that proteinuria during follow-up may not only be a result of broken-down renal tissue, but also a cause of decreased renal function; thus, prolonged exposure to proteinuria plays an important role in the development of kidney tissue disorder and its progression.
The increase in proteinuria course not only causes a decline in the renal function but is also a risk factor of CVD in patients with CKD [20, 21]. Clinicians should understand that follow-up evaluation of proteinuria is important in patients with BNS. More importantly, we revealed that despite the higher levels of proteinuria at baseline, the risk for reduced renal function decreases along with a decrease in the level of proteinuria during follow-up. Therefore, proper treatment by medication and reduction in urinary protein excretion are vital. In general, renin–angiotensin inhibitors and appropriate BP control can lower urinary protein excretion. However, in this study, the use of ACEis/ARBs and the values of BP were not associated with the TAP. Thus, we should reflect on unmeasured factors, which cause higher TAP values and a decline in kidney function. Salt intake is considered as one of the factors. In patients with CKD, the event of cardiovascular disease is related to the quantity of urinary sodium excretion [26], and the increase in salt intake promotes higher BP and proteinuria [27]. This is an important area for examination in future studies.
This study has several limitations. First, the findings cannot be generalized to other ethnic or age groups because the subjects analyzed were all inpatients in the university hospital. Moreover, in general, patients who are diagnosed with BNS barely undergo biopsies, while all patients in our study underwent biopsies. The possibility of the presence of sampling bias cannot be denied. Second, the number of participants was small, which also might present a limitation to the statistical power. Third, since this was a retrospective study, we could not analyze the causal relationship with treatment. Fourth, we did not use the proteinuria obtained via a 24-h urine collection. There might be a difference between proteinuria which was estimated by the spot urine protein–creatinine ratio and a 24-hurine collection. Also, we did not evaluate the impact on blood pressure control (during follow up) and additional ACEi/ARB use. In our study, almost all patients had good blood pressure control (126.6/77.9 mmHg on an average during follow-up) and had used ACEi/ARB during the follow-up (ACEi/ARB use was about 97% in total). Because of these, it was difficult to investigate the contribution of both blood pressure control and the use of ACEi/ARB. This is an important area for examination in future work. Finally, further studies are needed to assess whether other confounders, such as smoking history, serum calcium/phosphate, parathyroid hormone levels, tubular damage, and the thickness of renal artery, can affect the renal function and/or TAP [28]. Prospective studies on these relationships should be conducted to provide further insights.
Conclusion
In conclusion, an increase in TAP level is a risk factor for decline in renal function in patients with biopsy-proven BNS. Clinicians should understand that follow-up evaluation of proteinuria is important in patients with BNS. Further prospective studies are necessary to assess the association between TAP and reduced renal function.
Data availability
The datasets generated and analyzed during the study are not publicly available due to the terms of consent to which the participants agreed, but the datasets generated and/or analyzed during the current study are available upon request from the corresponding author.
References
Ruilope LM, van Veldhuisen DJ, Ritz E, Luscher TF. Renal function: the Cinderella of cardiovascular risk profile. J Am Coll Cardiol. 2001;38:1782–7.
Shoji T, Abe T, Matsuo H, Egusa G, Yamasaki Y, Kashihara N, et al. Chronic kidney disease, dyslipidemia, and atherosclerosis. J Atheroscler Thromb. 2012;19:299–315.
Iseki K, Ikemiya Y, Iseki C, Takishita S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003;63:1468–74.
Liyanage T, Ninomiya T, Jha V, Neal B, Patrice H, Okpechi I, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–82.
Ritz E. Minor renal dysfunction: an emerging independent cardiovascular risk factor. Heart. 2003;89:963–4.
Shiraishi N, Kitamura K, Kohda Y, Iseki K, Tomita K. Prevalence and risk factor analysis of nephrosclerosis and ischemic nephropathy in the Japanese general population. Clin Exp Nephrol. 2014;18:461–8.
Sumida K, Hoshino J, Ueno T, Mise K, Hayami N, Suwabe T, et al. Effect of proteinuria and glomerular filtration rate on renal outcome in patients with biopsy-proven benign nephrosclerosis. PLoS ONE. 2016;11:e0147690.
Barbour SJ, Cattran DC, Espino-Hernandez G, Hladunewich MA, Reich HN. Identifying the ideal metric of proteinuria as a predictor of renal outcome in idiopathic glomerulonephritis. Kidney Int. 2015;88:1392–401.
Reich HN, Troyanov S, Scholey JW, Cattran DC. The toronto glomerulonephritis registry. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18:3177–83.
Nishi S, Ubara Y, Utsunomiya Y, Okada K, Obata Y, Kai H, et al. Evidence-based clinical practice guidelines for nephrotic syndrome 2014. Clin Exp Nephrol. 2016;20:342–70.
Anonymous. Manual for pathological diagnosis of diabetic nephropathy and hypertensive nephrosclerosis. Nihon Jinzo Gakkai Shi. 2015;57:649–725. (in Japanese)
Vikse BE, Aasarød K, Bostad L, Iversen BM. Clinical prognostic factors in biopsy-proven benign nephrosclerosis. Nephrol Dial Transpl. 2003;18:517–23.
Kanda E, Usui T, Kashihara N, Iseki C, Iseki K, Nangaku M. Importance of glomerular filtration rate change as surrogate endpoint for the future incidence of end-stage renal disease in general Japanese population: community-based cohort study. Clin Exp Nephrol. 2018;22:318–27.
Altman DG, Bland JM. Diagnostic tests 3: receiver operating characteristic plots. BMJ. 1994;309:188.
Obuchowski NA, Lieber ML, Wians FH. ROC curves in clinical chemistry: uses, misuses, and possible solutions. Clin Chem. 2004;50:1118–25.
Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Matsuzaki K, Suzuki Y, Nakata J, Sakamoto N, Horikoshi S, Kawamura T, et al. Nationwide survey on current treatments for IgA nephropathy in Japan. Clin Exp Nephrol. 2013;17:827–33.
Kee YK, Yoon CY, Kim SJ, Moon SJ, Kim CH, Park JT, et al. Determination of the optimal target level of proteinuria in the management of patients with glomerular diseases by using different definitions of proteinuria. Medicine. 2017;96:e8154.
Momoki K, Kataoka H, Moriyama T, Mochizuki T, Nitta K. Hyperuricemia as a predictive marker for progression of nephrosclerosis: clinical assessment of prognostic factors in biopsy-proven arterial/arteriolar nephrosclerosis. J Atheroscler Thromb. 2017;24:630–42.
Haruhara K, Tsuboi N, Kanzaki G, Koike K, Suyama M, Shimizu A, et al. Glomerular density in biopsy-proven hypertensive nephrosclerosis. Am J Hypertens. 2015;28:1164–71.
Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81.
Bouchi R, Babazono T, Yoshida N, Nyumara I, Toya K, Hayashi T, et al. Association of albuminuria and reduced estimated glomerular filtration rate with incident stroke and coronary artery disease in patients with type 2 diabetes. Hypertens Res. 2010;33:1298–304.
Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–566.
Eddy AA, Giachelli CM. Renal expression of genes that promote interstitial inflammation and fibrosis in rats with protein-overload proteinuria. Kidney Int. 1995;47:1546–57.
Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974–84.
Mills KT, Chen J, Yang W, Appel LJ, Kusek JW, Alper A, et al. Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease. JAMA. 2016;315:2200–10.
Slagman MC, Waanders F, Hemmelder MH, Woittiez A-J, Janssen WMT, Lambers Heerspink HJ, et al. Moderate dietary sodium restriction added to angiotensin-converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: randomized controlled trial. BMJ. 2011;343:d4366.
Yamanouchi M, Hoshino J, Ubara Y, Takaichi K, Kinowaki K, Fujii T, et al. Clinicopathological predictors for progression of chronic kidney disease in nephrosclerosis: a biopsy-based cohort study. Nephrol Dial Transplant. 2018. https://doi.org/10.1093/ndt/gfy121.
Acknowledgements
We thank all staff, who were part of the Division of Nephrology and Hypertension, The Jikei University School of Medicine, for their comments and technical assistance.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at the Jikei University hospital (approval number 29-110), where the studies were conducted and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
All patients included in this study provided their written informed consent for performing the renal biopsy and analyzing the data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Amano, H., Koike, K., Haruhara, K. et al. Time-averaged proteinuria during follow-up and renal prognosis in patients with biopsy-proven benign nephrosclerosis. Clin Exp Nephrol 24, 688–695 (2020). https://doi.org/10.1007/s10157-020-01885-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-020-01885-w