Abstract
Background
Renal dysfunction is recognized with increasing frequency among the noninfectious comorbidities associated with human immunodeficiency virus (HIV) infection. Urinary liver-type fatty acid-binding protein (L-FABP) has been shown to be a new biomarker to screen for not only tubulointerstitial damage but also kidney dysfunction.
Methods
We performed a cross-sectional study to determine the association between the urinary L-FABP and chronic kidney disease (CKD) among 77 HIV-infected Japanese patients by backward-stepwise multivariable logistic regression.
Results
The prevalence of individuals in the low risk was 80 %. Urinary L-FABP level was not associated with antiretroviral therapy and tenofovir disoproxil fumarate. On the other hand, urinary L-FABP level was independently associated with the CKD classification.
Conclusion
Urinary L-FABP may be used as an adjunct to diagnose the CKD stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal dysfunction is recognized with increasing frequency among the noninfectious comorbidities associated with human immunodeficiency virus (HIV) infection, and is becoming a major cause of morbidity and mortality along with the declining incidence of acquired immune deficiency syndrome observed after the introduction of combination antiretroviral therapy (cART) [1–3]. The risks of end-stage renal disease, cardiovascular events and death increase in direct proportion to chronic kidney disease (CKD) stage [4], emphasizing the importance of identifying CKD in its early stages.
Liver-type fatty acid-binding protein (L-FABP) is expressed in the proximal tubules of the human kidney and participates in fatty acid metabolism [5–7]. Urinary L-FABP accurately reflected the severity of diabetic nephropathy in type 2 diabetes [8]. In type 1 diabetes mellitus (DM) patients, it was also reported that the level of urinary L-FABP was associated with albuminuria [9]. Additionally, it was described that urinary L-FABP might be a predictive marker for progression to end-stage renal disease and cardiovascular disease in type 2 diabetic patients and CKD patients without advanced nephropathy [10, 11]. Thus, urinary excretion of L-FABP was reported to offer potential as a clinical marker to screen for not only tubulointerstitial damage but also kidney dysfunction [12].
In HIV-infected patients, the studies of the prevalence of CKD in Japanese [13, 14] and urinary L-FABP secretion in patients receiving tenofovir disoproxil fumarate (TDF) [15] have been conducted. However, little is known regarding the association between the urinary L-FABP and CKD stage in HIV-infected patients. The aim of this study was to gain a more understanding of the clinical significance of urinary L-FABP.
Materials and methods
Study design and Patient population
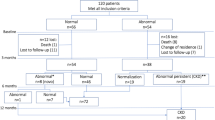
The pilot study was a cross-sectional design. A total of 229 HIV-infected patients were treated at The Hospital of Hyogo College of Medicine in Hyogo, Japan in 2013. We retrospectively reviewed their records and selected 77 Japanese patients with simultaneously obtained estimated glomerular filtration rate (eGFR), urinary protein or albumin, and L-FABP levels from among them. 149 patients were excluded because of lack of urinary L-FABP. Also, 3 patients who were not Japanese were excluded. The Ethics Review Board of Hyogo College of Medicine approved the study protocol (No. 2194).
Anthropometric and laboratory evaluation
We reviewed the electronic medical charts of all the subjects. Non-fasting blood and random urine samples were collected for analysis as part of routine clinical visits.
Biochemical data [creatinine, blood glucose, hemoglobin A1c (HbA1c)] and urinary beta2-microglobulin (β2MG) were measured by standard laboratory methods using an autoanalyzer for each patient. Urinary protein was measured as patients without DM. Urinary albumin was measured as patients with DM. Protein-to-creatinine ratio (PCR) was calculated by dividing urinary protein by urinary creatinine to predict 24-h proteinuria. Albumin to creatinine ratio (ACR) was calculated by dividing urinary albumin by urinary creatinine to predict 24-h albuminuria. eGFR was calculated as \({\text{eGFR}}\left( {{\text{mL/min/1}} . 7 3 {\text{m}}^{ 2} } \right) = 194 \times {\text{creatinine}}^{ - 1.094} \times {\text{age}}^{ - 0.287}\) in men, and \({\text{eGFR}}\left( {{\text{mL/min/1}} . 7 3 {\text{m}}^{2} } \right) = 194 \times {\text{creatinine}}^{ - 1.094} \times {\text{age}}^{ - 0.287} \times 0.739\) in women according to the criteria of the Japanese Society of Nephrology [16]. The HIV-RNA level was measured using the Cobas TaqMan HIV-1 real-time polymerase chain reaction version 2.0 assay (Roche Diagnostics, Branchburg, NJ, USA; lower detection limit, 20 copies/mL). Hypertension was defined as a systolic blood pressure of ≥140 mmHg and/or diastolic blood pressure of ≥90 mmHg, or the use of antihypertensive agents. DM was defined as a blood glucose of ≥200 mg/dL and HbA1c (NGSP) of ≥6.5 %, or the use of oral antidiabetic agents or insulin. Hepatitis C virus (HCV) infection was defined as a positive reactive HCV antibody test, while hepatitis B virus (HBV) infection was defined as a positive HBV surface antigen test. The urinary levels of L-FABP were measured using the enzyme-linked immunosorbent assay (Human L-FABP Assay Kit; CIMIC Co., Ltd., Tokyo, Japan), and were expressed as a ratio to urinary creatinine. The detection limits of the assays were 6 mg/dL for protein and 3 ng/mL for L-FABP. Concentrations below the lower detection limit were approximated using the mean value between zero and the lower detection limit, 3 mg/dL and 1.5 ng/mL, respectively.
Classification of CKD based on the GFR categories and proteinuria or albuminuria categories [17]
eGFR was classified into 6 grades—(G1) ≥90, (G2) 60–89, (G3a) 45–59, (G3b) 30–44, (G4) 15–29, and (G5) <15 mL/min/1.73 m2. PCR was classified into 3 grades—(A1) <0.15, (A2) 0.15–0.49, and (A3) ≥0.50 g/gCr, or ACR was classified into 3 grades—(A1) <30, (A2) 30–299, and (A3) ≥300 mg/gCr. The 6 eGFR and 3 PCR or ACR grades were classified into 4 risk zones for prognosis—low risk (G1A1, G2A1); moderately increased risk (G3aA1, G1A2, and G2A2); high risk (G3bA1, G3aA2, G1A3, and G2A3); and very high risk (G4A1, G5A1, G3bA2, G4A2, G5A2,G3aA3, G3bA3, G4A3, and G5A3).
Statistical methods
Categorical variables were compared between two groups using Fisher exact test or the χ 2 test. Differences between two groups were measured using the Mann–Whitney U test. Differences among three groups were measured using the Kruskal–Wallis test. The correlation between two variables was evaluated using Pearson’s correlation coefficient. Univariate analyses were conducted to screen independent variables using a liberal probability value of 0.10. Independent factors associated with CKD classification were determined using backward-stepwise multivariable logistic regression. A probability value <0.05 was considered significant. All analyses were conducted using SPSS software Windows version 14.0.
Results
Patient characteristics
Table 1 summarizes the demographic and clinical characteristics of individuals enrolled in this study. A total of 65 subjects (84 %) were receiving combination antiretroviral therapy, with 45 (69 %) administered TDF and 20 (31 %) administered abacavir. efavirenz, rilpivirine, a ritonavir-boosted protease inhibitor and raltegravir were used in 17 (26 %), 4 (6 %), 26 (40 %) and 20 (31 %) of the subjects, respectively. No patients used dolutegravir or cobicistat, the reducer of tubular secretion of creatinine. In 44 subjects, urinary L-FABP level was below the sensitivity of the assay. Urinary L-FABP level was determined, ranged from 0.6 to 70.9 μg/gCr.
Distribution by CKD classification based on the GFR and proteinuria
The results of the CKD classification are shown in Fig. 1. The prevalence of individuals in the low risk, the moderately increased risk, the high risk, and the very high risk zones was 80, 17, 3, and 0 %, respectively.
The association of urinary L-FABP level with β2MG, eGFR, proteinuria, cART, TDF, and CKD classification
Urinary levels of L-FABP were positively correlated with urinary levels of β2MG (r = 0.554, p < 0.001) (Fig. 2a). On the other hand, urinary levels of L-FABP were not correlated with proteinuria (r = 0.068, p = 0.571) (Fig. 2b) nor eGFR (r = −0.091, p = 0.429) (Fig. 2c). Urinary levels of L-FABP were not significantly associated with cART and TDF use (Tables 2, 3). The prevalence of the subjects with a higher level of urinary L-FABP (than upper limit of reference value of urinary L-FABP, 8.4 μg/gCr) in the low risk, the moderately increased risk, the high risk, and the very high risk zones were 4.8 % (3/62), 38.5 % (5/13), 0 % (0/2), and 0 % (0/0), respectively (Table 4).
The association of CKD classification with clinical characteristics
In univariate analysis, age (≥50 years old), receiving lipid lower therapy, hypertension, and urinary L-FABP level (≥8.4 μg/gCr) was significantly associated with CKD classification (p = 0.005, 0.012, 0.011, and 0.006, respectively) (Table 5). Multivariate logistic regression model was built and included the following variables: age, receiving lipid lower therapy, hypertension, eGFR, and urinary L-FABP. Age (≥50 years old) (p = 0.005) and urinary L-FABP level (≥8.4 μg/gCr) remained independently associated with urinary levels of L-FABP (≥8.4 μg/gCr) (Table 5).
Discussion
This is the first report regarding the association between the urinary levels of L-FABP and CKD classification in HIV-infected Japanese patients. Urinary levels of L-FABP independently associated with the CKD stage in the HIV-infected patients.
Of note, the prevalence of proteinuria (16 %) in this study was high, although the prevalence of proteinuria was 3.8–12.0 % by dipstick in the earlier study in Japan [13, 14]. Meanwhile, the prevalence of eGFR less than 60 mL/min/1.73 m2 (4 %) was similar to the earlier study [13, 14]. The difference between our and the earlier study [13, 14] may be due to the method for measurement of proteinuria. Siedner et al. [18] showed that dipstick had poor sensitivity in detecting low-grade proteinuria in HIV-infected patients. Masimango et al. [19] also showed the limited sensitivity and specificity of the dipstick to detect significant microalbuminuria. They demonstrated that the positive predictive value of positive urine dipstick was 15.4 % and the negative predictive value was 92.8 % [ 19 ]. Because dipstick mainly measures albumin concentration, it is likely that false-negative reaction by dipstick is occurred due to nonalbumin (tubular) proteinuria and HIV itself.
Urinary levels of L-FABP were positively correlated with not eGFR and proteinuria but urinary levels of β2MG. This result is supported by the study that L-FABP is located in proximal renal tubules [20]. However, Kamijo et al. indicated that urinary L-FABP had correlated not only the tubulointerstitial damage but also creatinine clearance and urinary protein [21, 22]. The difference between those and our results may be caused by the subjects: those inclusion criteria were patients with overt kidney disease.
The present study showed no significant association between urinary L-FABP and use of TDF. Jablonowska et al. [15] reported that risk factor of higher urinary levels of L-FABP was HIV/HCV coinfection with lower body weight in patients receiving TDF. Furthermore, Nishijima et al. [23, 24] showed that small body weight was identified as an independent risk factor for TDF-associated renal dysfunction. Because this study only included 2 HIV/HCV-coinfected subjects and we had no data of body weight, we did not analyze these factors.
Similar to the earlier report in type 2 diabetic patients [8], the present study demonstrated that urinary levels of L-FABP had independently reflected the CKD stage in HIV-infected patients. Peralta et al. [25] showed a J-shaped association between urinary levels of L-FABP and mortality in 908 HIV-infected women. Choi et al. [26] reported that CKD was associated with higher mortality risk. These studies support our results. Lucas GM et al. [27] showed that the risk factors of kidney disease in HIV-infected patients were suggested as older age, female sex, DM, hypertension, injection drug use, lower CD4 cell count, specific antiretroviral drugs, history of acute kidney injury, and higher HIV-RNA levels. The different result might be caused by sample size, especially about female sex, DM, past history of acute kidney injury and lower CD4 cell count. The amount of β2MG excreted in urine is affected mainly by condition of the tubules [28, 29]. Therefore, urinary levels of β2MG were not associated with CKD classification.
Urinary levels of L-FABP may be used as an adjunct to diagnose the CKD stage. Although correctly estimating glomerular filtration rate is essential for staging of CKD, some antiretroviral drugs and pharmacokinetics enhancers apparently reduce eGFR based on serum creatinine. Dolutegravir, which inhibits mainly the organic cation transporter 2, and cobicistat, which predominantly inhibits the multidrug and toxin extrusion protein 1, decrease tubular secretion of creatinine and therefore increase concentrations of serum creatinine without affecting actual glomerular filtration [30, 31]. Although cystatin C is also one of the urinary biomarkers, we have clarified that eGFR based on cystatin C was underestimated in HIV-infected patients with HIV-RNA ≥500 copies/mL [32].
Our study has several limitations. First, our study was cross-sectional; therefore, it is difficult to determine the association of the development of chronic kidney disease. Second, over half of subjects in this study could not determine accurate urinary levels of L-FABP since lower detection limit of urinary levels of L-FABP was 3.0 ng/mL. We are investigating the data measured by higher sensitivity of urinary levels of L-FABP: lower detection limit, 1.5 ng/mL. Third, this study population comprised mainly HIV-infected Japanese men without overt renal dysfunction. Accordingly, the results may not be generalizable to women or patients with moderate-to-severe kidney disease. Nonetheless, our results are worthwhile because most of HIV-infected patients have mild CKD and it is important to identify CKD in its early stages.
Conclusion
The present study demonstrates that urinary levels of L-FABP are independently associated with the CKD stage in HIV-infected patients. Urinary L-FABP may be used as an adjunct to diagnose the CKD stage.
References
Palella FJ Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr (1999). 2006;43(1):27–34. doi:10.1097/01.qai.0000233310.90484.16.
Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–60. doi:10.1056/nejm199803263381301.
Roling J, Schmid H, Fischereder M, Draenert R, Goebel FD. HIV-associated renal diseases and highly active antiretroviral therapy-induced nephropathy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2006;42(10):1488–95. doi:10.1086/503566.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. doi:10.1056/NEJMoa041031.
Sweetser DA, Heuckeroth RO, Gordon JI. The metabolic significance of mammalian fatty-acid-binding proteins: abundant proteins in search of a function. Annu Rev Nutr. 1987;7:337–59. doi:10.1146/annurev.nu.07.070187.002005.
Veerkamp JH, Peeters RA, Maatman RG. Structural and functional features of different types of cytoplasmic fatty acid-binding proteins. Biochim Biophys Acta. 1991;1081(1):1–24.
Veerkamp JH, van Kuppevelt TH, Maatman RG, Prinsen CF. Structural and functional aspects of cytosolic fatty acid-binding proteins. Prostaglandins Leukot Essent Fat Acids. 1993;49(6):887–906.
Kamijo-Ikemori A, Sugaya T, Yasuda T, Kawata T, Ota A, Tatsunami S, et al. Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care. 2011;34(3):691–6. doi:10.2337/dc10-1392.
Nielsen SE, Sugaya T, Tarnow L, Lajer M, Schjoedt KJ, Astrup AS, et al. Tubular and glomerular injury in diabetes and the impact of ACE inhibition. Diabetes Care. 2009;32(9):1684–8. doi:10.2337/dc09-0429.
Araki S, Haneda M, Koya D, Sugaya T, Isshiki K, Kume S, et al. Predictive effects of urinary liver-type fatty acid-binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy. Diabetes Care. 2013;36(5):1248–53. doi:10.2337/dc12-1298.
Matsui K, Kamijo-Ikemori A, Imai N, Sugaya T, Yasuda T, Tatsunami S, et al. Clinical significance of urinary liver-type fatty acid-binding protein as a predictor of ESRD and CVD in patients with CKD. Clin Exp Nephrol. 2016;20(2):195–203. doi:10.1007/s10157-015-1144-9.
Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hikawa A, Hirano N, et al. Urinary fatty acid-binding protein as a new clinical marker of the progression of chronic renal disease. J Lab Clin Med. 2004;143(1):23–30. doi:10.1016/s0022214303001872.
Muramatsu T, Yanagisawa N, Chikasawa Y, Seita I, Yotsumoto M, Otaki M, et al. Prevalence of chronic kidney disease among HIV-infected individuals in Japan–a report from two tertiary hospitals. Kansenshogaku zasshi. J Jpn Assoc Infect Dis. 2013;87(1):14–21.
Yanagisawa N, Muramatsu T, Yamamoto Y, Tsuchiya K, Nitta K, Ajisawa A, et al. Classification of human immunodeficiency virus-infected patients with chronic kidney disease using a combination of proteinuria and estimated glomerular filtration rate. Clin Exp Nephrol. 2014;18(4):600–5. doi:10.1007/s10157-013-0853-1.
Jablonowska E, Wojcik K, Piekarska A. Urine liver-type fatty acid-binding protein and kidney injury molecule-1 in HIV-infected patients receiving combined antiretroviral treatment based on tenofovir. AIDS Res Hum Retrovir. 2014;30(4):363–9. doi:10.1089/AID.2013.0070.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis Off J Natl Kidney Found. 2009;53(6):982–92. doi:10.1053/j.ajkd.2008.12.034.
Japan nephrology s. Special issue: Clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012. Nihon Jinzo Gakkai shi. 2012;54(8):1034–191.
Siedner MJ, Atta MG, Lucas GM, Perazella MA, Fine DM. Poor validity of urine dipstick as a screening tool for proteinuria in HIV-positive patients. J Acquir Immune Def Syndr (1999). 2008;47(2):261–3. doi:10.1097/QAI.0b013e31815ac4ad.
Masimango MI, Sumaili EK, Jadoul M, Wallemacq P, Mubagwa DK, Makulo RJ, et al. Prevalence of microalbuminuria and diagnostic value of dipstick proteinuria in outpatients from HIV clinics in Bukavu, the Democratic Republic of Congo. BMC Nephrol. 2014;15:146. doi:10.1186/1471-2369-15-146.
Pelsers MM. Fatty acid-binding protein as marker for renal injury. Scand J Clin Lab Invest Suppl. 2008;241:73–7. doi:10.1080/00365510802150133.
Kamijo A, Sugaya T, Hikawa A, Okada M, Okumura F, Yamanouchi M, et al. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am J Pathol. 2004;165(4):1243–55. doi:10.1016/s0002-9440(10)63384-6.
Kamijo A, Sugaya T, Hikawa A, Yamanouchi M, Hirata Y, Ishimitsu T, et al. Clinical evaluation of urinary excretion of liver-type fatty acid-binding protein as a marker for the monitoring of chronic kidney disease: a multicenter trial. J Lab Clin Med. 2005;145(3):125–33.
Nishijima T, Gatanaga H, Komatsu H, Tsukada K, Shimbo T, Aoki T, et al. Renal function declines more in tenofovir- than abacavir-based antiretroviral therapy in low-body weight treatment-naive patients with HIV infection. PLoS One. 2012;7(1):e29977. doi:10.1371/journal.pone.0029977.
Nishijima T, Komatsu H, Gatanaga H, Aoki T, Watanabe K, Kinai E, et al. Impact of small body weight on tenofovir-associated renal dysfunction in HIV-infected patients: a retrospective cohort study of Japanese patients. PLoS One. 2011;6(7):e22661. doi:10.1371/journal.pone.0022661.
Peralta C, Scherzer R, Grunfeld C, Abraham A, Tien P, Devarajan P, et al. Urinary biomarkers of kidney injury are associated with all-cause mortality in the Women’s Interagency HIV Study (WIHS). HIV Med. 2014;15(5):291–300. doi:10.1111/hiv.12113.
Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Volberding PA, O’Hare AM. The impact of HIV on chronic kidney disease outcomes. Kidney Int. 2007;72(11):1380–7.
Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;59(9):e96–138. doi:10.1093/cid/ciu617.
Hall PW 3rd, Dammin GJ. Balkan nephropathy. Nephron. 1978;22(4–6):281–300.
Taniguchi N, Tanaka M, Kishihara C, Ohno H, Kondo T, Matsuda I, et al. Determination of carbonic anhydrase C and beta 2-microglobulin by radioimmunoassay in urine of heavy-metal-exposed subjects and patients with renal tubular acidosis. Environ Res. 1979;20(1):154–61.
German P, Liu HC, Szwarcberg J, Hepner M, Andrews J, Kearney BP, et al. Effect of cobicistat on glomerular filtration rate in subjects with normal and impaired renal function. J Acquir Immune Def Syndr (1999). 2012;61(1):32–40. doi:10.1097/QAI.0b013e3182645648.
Koteff J, Borland J, Chen S, Song I, Peppercorn A, Koshiba T, et al. A phase 1 study to evaluate the effect of dolutegravir on renal function via measurement of iohexol and para-aminohippurate clearance in healthy subjects. Br J Clin Pharmacol. 2013;75(4):990–6. doi:10.1111/j.1365-2125.2012.04440.x.
Hikasa S, Yasuda M, Hideta K, Kimura T, Sawada A, Tokugawa T, et al. Comparison between estimated glomerular filtration rate based on cystatin C and estimated glomerular filtration rate based on creatinine in Japanese HIV-infected male patients. J AIDS Res. 2016;18:13–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has conflict of interest with the submission.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee at which the studies conducted (IRB Approval No. 2194) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
We obtained consent through opt-out procedure from all individual participants included in the study.
About this article
Cite this article
Hikasa, S., Yasuda, M., Hideta, K. et al. The association between urinary liver-type fatty acid-binding protein and chronic kidney disease classification in HIV-infected Japanese patients. Clin Exp Nephrol 21, 971–977 (2017). https://doi.org/10.1007/s10157-016-1347-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-016-1347-8