Abstract
Background
Secondary hyperparathyroidism (SHPT) is common in end-stage renal disease. Our primary objective was to evaluate the efficacy of oral paricalcitol versus oral calcitriol on serum intact parathyroid hormone (iPTH) and mineral bone parameters in continuous ambulatory peritoneal dialysis (CAPD) patients with SHPT. The secondary objective was to analyze highly sensitive C-reactive protein (hsCRP) and peritoneal membrane function in both groups.
Methods
This was a prospective randomized control trial. CAPD patients with SHPT were randomized to paricalcitol or calcitriol for 15 weeks. Serum intact iPTH, calcium, phosphate and alkaline phosphatase (ALP) were measured at baseline and every 3 weeks. Serum hsCRP and peritoneal membrane functions were measured at baseline and at week 15.
Results
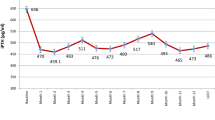
A total of 26 patients were enrolled and randomized—12 to paricalcitol and 14 to calcitriol. Serum iPTH reduced significantly in both groups and there was no difference in the incidence of ≥50 % reduction of iPTH between both groups. There was a significant increase in serum calcium in both groups but there were no differences in serum phosphorus across the visits. The incidence of hypercalcemia was the same in both groups. Serum calcium–phosphorus (Ca × P) product increased in the paricalcitol group but decreased in the calcitriol group. Serum ALP decreased significantly in both groups. There were also no differences in pre- and post-treatment serum hsCRP and peritoneal function test (PFT) in both groups.
Conclusion
Both oral paricalcitol and calcitriol were equally efficacious in reducing serum iPTH but were associated with significantly higher serum calcium. Serum Ca × P product increased in the paricalcitol group and decreased in the calcitriol group. Serum hsCRP level and PFT were not affected by either treatment. A larger randomized controlled trial is indicated to confirm these initial findings.






Similar content being viewed by others
References
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int. 2009;76(Suppl 113):S50–99.
Couttenye MM, D’Haese PC, Van Hoof VO, Lemoniatou E, Goodman W, Verpooten GA, et al. Low serum levels of alkaline phosphatase of bone origin: a good marker of adynamic bone disease in haemodialysis patients. Nephrol Dial Transplant. 1996;11(6):1065–72.
Ritz E, Schomig M, Bommer J. Osteodystrophy in the millennium. Kidney Int. 1999;73(Suppl):S94–8.
Khan S. Vitamin D deficiency and secondary hyperparathyroidism among patients with chronic kidney disease. Am J Med Sci. 2007;333(4):201–7.
Yudd M, Llach F. Current medical management of secondary hyperparathyroidism. Am J Med Sci. 2000;320(2):100–6.
Davies MR, Hruska KA. Pathophysiological mechanisms of vascular calcification in end-stage renal disease. Kidney Int. 2001;60(2):472–9.
Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342(20):1478–83.
Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62(1):245–52.
Milliner DS, Zinsmeister AR, Lieberman E, Landing B. Soft tissue calcification in pediatric patients with end-stage renal disease. Kidney Int. 1990;38(5):931–6.
Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca × PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12(10):2131–8.
Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium X phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–17.
Sprague SM, Lerma E, McCormmick D, Abraham M, Batlle D. Suppression of parathyroid hormone secretion in hemodialysis patients: comparison of paricalcitol with calcitriol. Am J Kidney Dis. 2001;38(Suppl 5):S51–6.
Olafur SI, Darryl LQ. Comparison of treatment for mild secondary hyperparathyroidism in haemodialysis patients. Kidney Int. 2000;57:282–92.
Yakahashi F, Finch JL, Denda M, Dusso AS, Brown AJ, Slatopolsky E. A new analog of 1,25(OH)2D2, 19-nor-1,25(OH)2D2, suppresses serum PTH and parathyroid gland growth in uremic rats without elevation of intestinal vitamin D receptor content. Am J Kidney Dis. 1997;30:105–12.
Brown AJ, Finch J, Slatopolsky E. Differential effects of 19-nor-1, 25-(OH)2D2 and 1 a-hydroxyvitamin D3 on the intestinal calcium and phosphate transport. J Lab Clin Med. 2002;139:279–84.
Abdul Gafor AH, Saidin R, Loo CY, Mohd R, Zainudin S, Shah SA, Norella KC. Intravenous calcitriol versus paricalcitol in haemodialysis patients with severe secondary hyperparathyroidism. Nephrology (Carlton). 2009;14(5):488–92.
Parikh NI, Hwang SJ, Larson MG, Meigs JB, Levy D, Fox CS. Cardiovascular disease risk factors in chronic kidney disease: overall burden and rates of treatment and control. Arch Intern Med. 2006;166(17):1884–91.
Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95(12):787–96.
Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96(6):1755–60.
London GM, Guerin AP, Verbeke FH, et al. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18(2):613–20.
Ross EA, Tian J, Abboud H, et al. Oral paricalcitol for the treatment of secondary hyperparathyroidism in patients on hemodialysis or peritoneal dialysis. Am J Nephrol. 2008;28(1):97–106.
Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003;63(4):1483–90.
Kurz P, Roth P, Werner E, Vlachojannis J, Grutzmacher P. Factors influencing transperitoneal calcium balance during CAPD. ASAIO J. 1992;38(3):589–92.
Simonsen O, Venturoli D, Wieslander A, Carlsson O, Rippe B. Mass transfer of calcium across the peritoneum at three different peritoneal dialysis fluid Ca2+ and glucose concentrations. Kidney Int. 2003;64(1):208–15.
Llach F, Yudd M. Paricalcitol in dialysis patients with calcitriol-resistant secondary hyperparathyroidism. Am J Kidney Dis. 2001;38(Suppl 5):S45–50.
Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–18.
Massry SG, Coburn JW, Lee DB, Jowsey J, Kleeman CR. Clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(Suppl 3):S1–201.
Cozzolino M, Dusso AS, Slatoplolsky E. Role of calcium–phosphate product and bone-associated proteins on vascular calcification in renal failure. J Am Soc Nephrol. 2001;12:2511–6.
Ha SK, Park CH, Seo JK, et al. Studies on bone markers and bone mineral density in patients with chronic renal failure. Yonsei Med J. 1996;37(5):350–6.
Martin KJ, Gonzalez EA, Gellens M, Hamm LL, Abboud H, Lindberg J. 19-Nor-1-alpha-25-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol. 1998;9(8):1427–32.
Noorul Afidza M, Ruslinda M, Norazinizah AM, et al. Serum vitamin D levels in normal subjects and patients with CKD [abstract]. In: Proceeding of the 27th Annual Congress of Malaysia Society of Nephrology, Kuala Lumpur, Malaysia, 6–8 May 2011. Abstract OP01: p 82.
Becker LE, Koleganova N, Piecha G, et al. Effect of paricalcitol and calcitriol on aortic wall remodeling in uninephrectomized ApoE knockout mice. Am J Physiol Renal Physiol. 2011;300:772–82.
Izquierdo MJ, Cavia M, Muñiz P, et al. Paricalcitol reduces oxidative stress and inflammation in hemodialysis patients. BMC Nephrol. 2012;13:159.
Guillot X, Semerano L, Saidenberg-Kermanac’h N, Falgarone G, Boissier MC. Vitamin D and inflammation. Jt Bone Spine. 2010;77:552–7.
Ruslinda M, Rozita M, Norella Kong CT, et al. Effects of calcitriol supplement on serum levels of inflammatory biomarkers in CKD patients with hypovitaminosis D. In: Proceeding of the 27th Annual Congress of Malaysia Society of Nephrology, Kuala Lumpur, Malaysia, 6–8 May 2011. Abstract OP018: p 83.
Chung SH, Heimbürger O, Stenvinkel P, Bergström J, Lindholm B. Association between inflammation and changes in residual renal function and peritoneal transport rate during the first year of dialysis. Nephrol Dial Transplant. 2001;16(11):2240–5.
Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers and the risk of incident hypertension. Hypertension. 2007;49(3):432–8.
Acknowledgments
This study was supported by the Faculty of Medicine UKM Research Grant FF-262-2010.
Conflict of interest
The authors have declared that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Jamaluddin, E.J., Gafor, A.H.A., Yean, L.C. et al. Oral paricalcitol versus oral calcitriol in continuous ambulatory peritoneal dialysis patients with secondary hyperparathyroidism. Clin Exp Nephrol 18, 507–514 (2014). https://doi.org/10.1007/s10157-013-0844-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-013-0844-2