Abstract
Artificial intelligence (AI) has the potential to revolutionize surgery in the coming years. Still, it is essential to clarify what the meaningful current applications are and what can be reasonably expected. This AI-powered review assessed the role of AI in colorectal surgery. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-compliant systematic search of PubMed, Embase, Scopus, Cochrane Library databases, and gray literature was conducted on all available articles on AI in colorectal surgery (from January 1 1997 to March 1 2021), aiming to define the perioperative applications of AI. Potentially eligible studies were identified using novel software powered by natural language processing (NLP) and machine learning (ML) technologies dedicated to systematic reviews. Out of 1238 articles identified, 115 were included in the final analysis. Available articles addressed the role of AI in several areas of interest. In the preoperative phase, AI can be used to define tailored treatment algorithms, support clinical decision-making, assess the risk of complications, and predict surgical outcomes and survival. Intraoperatively, AI-enhanced surgery and integration of AI in robotic platforms have been suggested. After surgery, AI can be implemented in the Enhanced Recovery after Surgery (ERAS) pathway. Additional areas of applications included the assessment of patient-reported outcomes, automated pathology assessment, and research. Available data on these aspects are limited, and AI in colorectal surgery is still in its infancy. However, the rapid evolution of technologies makes it likely that it will increasingly be incorporated into everyday practice.



Similar content being viewed by others
References
Lam K, Abramoff MD, Balibrea JM, Bishop SM, Brady RR, Callcut RA, Chand M, Collins JW, Diener MK, Eisenmann M, Fermont K, Neto MG, Hager GD, Hinchliffe RJ, Horgan A, Jannin P, Langerman A, Logishetty K, Mahadik A, Maier-Hein L, Antona EM, Mascagni P, Mathew RK, Muller-Stich BP, Neumuth T, Nickel F, Park A, Pellino G, Rudzicz F, Shah S, Slack M, Smith MJ, Soomro N, Speidel S, Stoyanov D, Tilney HS, Wagner M, Darzi A, Kinross JM, Purkayastha S (2022) A Delphi consensus statement for digital surgery. NPJ Digit Med 5(1):100. https://doi.org/10.1038/s41746-022-00641-6
Copeland B (2020) Artificial intelligence. Encyclopedia Britannica. https://www.britannica.com/technology/artificial-intelligence. Accessed 16 Sep 2022
Pandey B, Mishra RB (2009) Knowledge and intelligent computing system in medicine. Comput Biol Med 39(3):215–230. https://doi.org/10.1016/j.compbiomed.2008.12.008
El Hechi M, Ward TM, An GC, Maurer LR, El Moheb M, Tsoulfas G, Kaafarani HM (2021) Artificial intelligence, machine learning, and surgical science: reality versus hype. J Surg Res 264:A1–A9. https://doi.org/10.1016/j.jss.2021.01.046
Bhandari M, Zeffiro T, Reddiboina M (2020) Artificial intelligence and robotic surgery: current perspective and future directions. Curr Opin Urol 30(1):48–54. https://doi.org/10.1097/MOU.0000000000000692
Rimmer L, Howard C, Picca L, Bashir M (2021) The automaton as a surgeon: the future of artificial intelligence in emergency and general surgery. Eur J Trauma Emerg Surg 47(3):757–762. https://doi.org/10.1007/s00068-020-01444-8
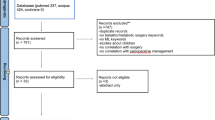
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev 5(1):210. https://doi.org/10.1186/s13643-016-0384-4
Pourahmad S, Pourhashemi S, Mohammadianpanah M (2016) Colorectal cancer staging using three clustering methods based on preoperative clinical findings. Asian Pac J Cancer Prev 17(2):823–827. https://doi.org/10.7314/apjcp.2016.17.2.823
Maheshwari K, Cywinski J, Mathur P, Cummings KC 3rd, Avitsian R, Crone T, Liska D, Campion FX, Ruetzler K, Kurz A (2019) Identify and monitor clinical variation using machine intelligence: a pilot in colorectal surgery. J Clin Monit Comput 33(4):725–731. https://doi.org/10.1007/s10877-018-0200-x
Curtis NJ, Dennison G, Salib E, Hashimoto DA, Francis NK (2019) Artificial neural network individualised prediction of time to colorectal cancer surgery. Gastroenterol Res Pract 2019:1285931. https://doi.org/10.1155/2019/1285931
Gao F, Wang W, Tan M, Zhu L, Zhang Y, Fessler E, Vermeulen L, Wang X (2019) DeepCC: a novel deep learning-based framework for cancer molecular subtype classification. Oncogenesis 8(9):44. https://doi.org/10.1038/s41389-019-0157-8
Park JH, Baek JH, Sym SJ, Lee KY, Lee Y (2020) A data-driven approach to a chemotherapy recommendation model based on deep learning for patients with colorectal cancer in Korea. BMC Med Inform Decis Mak 20(1):241. https://doi.org/10.1186/s12911-020-01265-0
Aikemu B, Xue P, Hong H, Jia H, Wang C, Li S, Huang L, Ding X, Zhang H, Cai G, Lu A, Xie L, Li H, Zheng M, Sun J (2020) Artificial intelligence in decision-making for colorectal cancer treatment strategy: an observational study of implementing Watson for oncology in a 250-case cohort. Front Oncol 10:594182. https://doi.org/10.3389/fonc.2020.594182
Kim EJ, Woo HS, Cho JH, Sym SJ, Baek JH, Lee WS, Kwon KA, Kim KO, Chung JW, Park DK, Kim YJ (2019) Early experience with Watson for oncology in Korean patients with colorectal cancer. PLoS ONE 14(3):e0213640. https://doi.org/10.1371/journal.pone.0213640
Chan HC, Chattopadhyay A, Chuang EY, Lu TP (2021) Development of a gene-based prediction model for recurrence of colorectal cancer using an ensemble learning algorithm. Front Oncol 11:631056. https://doi.org/10.3389/fonc.2021.631056
Stromblad CT, Baxter-King RG, Meisami A, Yee SJ, Levine MR, Ostrovsky A, Stein D, Iasonos A, Weiser MR, Garcia-Aguilar J, Abu-Rustum NR, Wilson RS (2021) Effect of a predictive model on planned surgical duration accuracy, patient wait time, and use of presurgical resources: a randomized clinical trial. JAMA Surg 156(4):315–321. https://doi.org/10.1001/jamasurg.2020.6361
Azimi K, Honaker MD, Chalil Madathil S, Khasawneh MT (2020) Post-operative infection prediction and risk factor analysis in colorectal surgery using data mining techniques: a pilot study. Surg Infect (Larchmt) 21(9):784–792. https://doi.org/10.1089/sur.2019.138
Choi J, Bessoff K, Bromley-Dulfano R, Li Z, Gupta A, Taylor K, Wadhwa H, Seltzer R, Spain DA, Knowlton LM (2021) Prospectively assigned AAST grade versus modified Hinchey class and acute diverticulitis outcomes. J Surg Res 259:555–561. https://doi.org/10.1016/j.jss.2020.10.016
Manilich E, Remzi FH, Fazio VW, Church JM, Kiran RP (2012) Prognostic modeling of preoperative risk factors of pouch failure. Dis Colon Rectum 55(4):393–399. https://doi.org/10.1097/DCR.0b013e3182452594
Sammour T, Cohen L, Karunatillake AI, Lewis M, Lawrence MJ, Hunter A, Moore JW, Thomas ML (2017) Validation of an online risk calculator for the prediction of anastomotic leak after colon cancer surgery and preliminary exploration of artificial intelligence-based analytics. Tech Coloproctol 21(11):869–877. https://doi.org/10.1007/s10151-017-1701-1
Francis NK, Luther A, Salib E, Allanby L, Messenger D, Allison AS, Smart NJ, Ockrim JB (2015) The use of artificial neural networks to predict delayed discharge and readmission in enhanced recovery following laparoscopic colorectal cancer surgery. Tech Coloproctol 19(7):419–428. https://doi.org/10.1007/s10151-015-1319-0
PelvEx C (2020) Predicting outcomes of pelvic exenteration using machine learning. Colorectal Dis 22(12):1933–1940. https://doi.org/10.1111/codi.15235
Joshi N, Bond S, Brady M (2010) The segmentation of colorectal MRI images. Med Image Anal 14(4):494–509. https://doi.org/10.1016/j.media.2010.03.002
Antunes JT, Ofshteyn A, Bera K, Wang EY, Brady JT, Willis JE, Friedman KA, Marderstein EL, Kalady MF, Stein SL, Purysko AS, Paspulati R, Gollamudi J, Madabhushi A, Viswanath SE (2020) Radiomic features of primary rectal cancers on baseline T2 -weighted MRI are associated with pathologic complete response to neoadjuvant chemoradiation: a multisite study. J Magn Reson Imaging 52(5):1531–1541. https://doi.org/10.1002/jmri.27140
Alvarez-Jimenez C, Antunes JT, Talasila N, Bera K, Brady JT, Gollamudi J, Marderstein E, Kalady MF, Purysko A, Willis JE, Stein S, Friedman K, Paspulati R, Delaney CP, Romero E, Madabhushi A, Viswanath SE (2020) Radiomic texture and shape descriptors of the rectal environment on post-chemoradiation T2-weighted MRI are associated with pathologic tumor stage regression in rectal cancers: a retrospective Multi-Institution Study. Cancers (Basel). https://doi.org/10.3390/cancers12082027
Yuan Z, Xu T, Cai J, Zhao Y, Cao W, Fichera A, Liu X, Yao J, Wang H (2022) Development and validation of an image-based deep learning algorithm for detection of synchronous peritoneal carcinomatosis in colorectal cancer. Ann Surg 275(4):e645–e651. https://doi.org/10.1097/SLA.0000000000004229
Liang M, Cai Z, Zhang H, Huang C, Meng Y, Zhao L, Li D, Ma X, Zhao X (2019) Machine learning-based analysis of rectal cancer mri radiomics for prediction of metachronous liver metastasis. Acad Radiol 26(11):1495–1504. https://doi.org/10.1016/j.acra.2018.12.019
Meng X, Xia W, Xie P, Zhang R, Li W, Wang M, Xiong F, Liu Y, Fan X, Xie Y, Wan X, Zhu K, Shan H, Wang L, Gao X (2019) Preoperative radiomic signature based on multiparametric magnetic resonance imaging for noninvasive evaluation of biological characteristics in rectal cancer. Eur Radiol 29(6):3200–3209. https://doi.org/10.1007/s00330-018-5763-x
Li M, Zhang J, Dan Y, Yao Y, Dai W, Cai G, Yang G, Tong T (2020) A clinical-radiomics nomogram for the preoperative prediction of lymph node metastasis in colorectal cancer. J Transl Med 18(1):46. https://doi.org/10.1186/s12967-020-02215-0
Liu M, Ma X, Shen F, Xia Y, Jia Y, Lu J (2020) MRI-based radiomics nomogram to predict synchronous liver metastasis in primary rectal cancer patients. Cancer Med 9(14):5155–5163. https://doi.org/10.1002/cam4.3185
Maaref A, Romero FP, Montagnon E, Cerny M, Nguyen B, Vandenbroucke F, Soucy G, Turcotte S, Tang A, Kadoury S (2020) Predicting the response to FOLFOX-based chemotherapy regimen from untreated liver metastases on baseline CT: a deep neural network approach. J Digit Imaging 33(4):937–945. https://doi.org/10.1007/s10278-020-00332-2
Nakanishi R, Akiyoshi T, Toda S, Murakami Y, Taguchi S, Oba K, Hanaoka Y, Nagasaki T, Yamaguchi T, Konishi T, Matoba S, Ueno M, Fukunaga Y, Kuroyanagi H (2020) Radiomics approach outperforms diameter criteria for predicting pathological lateral lymph node metastasis after neoadjuvant (chemo)radiotherapy in advanced low rectal cancer. Ann Surg Oncol 27(11):4273–4283. https://doi.org/10.1245/s10434-020-08974-w
Lee S, Choe EK, Kim SY, Kim HS, Park KJ, Kim D (2020) Liver imaging features by convolutional neural network to predict the metachronous liver metastasis in stage I-III colorectal cancer patients based on preoperative abdominal CT scan. BMC Bioinform 21(Suppl 13):382. https://doi.org/10.1186/s12859-020-03686-0
Maier-Hein L, Wagner M, Ross T, Reinke A, Bodenstedt S, Full PM, Hempe H, Mindroc-Filimon D, Scholz P, Tran TN, Bruno P, Kisilenko A, Muller B, Davitashvili T, Capek M, Tizabi MD, Eisenmann M, Adler TJ, Grohl J, Schellenberg M, Seidlitz S, Lai TYE, Pekdemir B, Roethlingshoefer V, Both F, Bittel S, Mengler M, Mundermann L, Apitz M, Kopp-Schneider A, Speidel S, Nickel F, Probst P, Kenngott HG, Muller-Stich BP (2021) Heidelberg colorectal data set for surgical data science in the sensor operating room. Sci Data 8(1):101. https://doi.org/10.1038/s41597-021-00882-2
Kitaguchi D, Takeshita N, Matsuzaki H, Oda T, Watanabe M, Mori K, Kobayashi E, Ito M (2020) Automated laparoscopic colorectal surgery workflow recognition using artificial intelligence: experimental research. Int J Surg 79:88–94. https://doi.org/10.1016/j.ijsu.2020.05.015
Kitaguchi D, Takeshita N, Matsuzaki H, Takano H, Owada Y, Enomoto T, Oda T, Miura H, Yamanashi T, Watanabe M, Sato D, Sugomori Y, Hara S, Ito M (2020) Real-time automatic surgical phase recognition in laparoscopic sigmoidectomy using the convolutional neural network-based deep learning approach. Surg Endosc 34(11):4924–4931. https://doi.org/10.1007/s00464-019-07281-0
Baltussen EJM, Snaebjornsson P, de Koning SGB, Sterenborg H, Aalbers AGJ, Kok N, Beets GL, Hendriks BHW, Kuhlmann KFD, Ruers TJM (2017) Diffuse reflectance spectroscopy as a tool for real-time tissue assessment during colorectal cancer surgery. J Biomed Opt 22(10):1–6. https://doi.org/10.1117/1.JBO.22.10.106014
Baltussen EJM, Brouwer de Koning SG, Sanders J, Aalbers AGJ, Kok NFM, Beets GL, Hendriks BHW, Sterenborg H, Kuhlmann KFD, Ruers TJM (2019) Tissue diagnosis during colorectal cancer surgery using optical sensing: an in vivo study. J Transl Med 17(1):333. https://doi.org/10.1186/s12967-019-2083-0
Baltussen EJM, Brouwer de Koning SG, Sanders J, Aalbers AGJ, Kok NFM, Beets GL, Hendriks BHW, Sterenborg H, Kuhlmann KFD, Ruers TJM (2020) Using diffuse reflectance spectroscopy to distinguish tumor tissue from fibrosis in rectal cancer patients as a guide to surgery. Lasers Surg Med 52(7):604–611. https://doi.org/10.1002/lsm.23196
Cahill RA, O’Shea DF, Khan MF, Khokhar HA, Epperlein JP, Mac Aonghusa PG, Nair R, Zhuk SM (2021) Artificial intelligence indocyanine green (ICG) perfusion for colorectal cancer intra-operative tissue classification. Br J Surg 108(1):5–9. https://doi.org/10.1093/bjs/znaa004
Park SH, Park HM, Baek KR, Ahn HM, Lee IY, Son GM (2020) Artificial intelligence based real-time microcirculation analysis system for laparoscopic colorectal surgery. World J Gastroenterol 26(44):6945–6962. https://doi.org/10.3748/wjg.v26.i44.6945
Shademan A, Decker RS, Opfermann JD, Leonard S, Krieger A, Kim PC (2016) Supervised autonomous robotic soft tissue surgery. Sci Transl Med 8(337):337. https://doi.org/10.1126/scitranslmed.aad9398
Weller GB, Lovely J, Larson DW, Earnshaw BA, Huebner M (2018) Leveraging electronic health records for predictive modeling of post-surgical complications. Stat Methods Med Res 27(11):3271–3285. https://doi.org/10.1177/0962280217696115
Adams K, Papagrigoriadis S (2014) Creation of an effective colorectal anastomotic leak early detection tool using an artificial neural network. Int J Colorectal Dis 29(4):437–443. https://doi.org/10.1007/s00384-013-1812-8
Sohn S, Larson DW, Habermann EB, Naessens JM, Alabbad JY, Liu H (2017) Detection of clinically important colorectal surgical site infection using Bayesian network. J Surg Res 209:168–173. https://doi.org/10.1016/j.jss.2016.09.058
Blansit K, Marmor R, Zhao B, Tien D (2017) Voice enabled framework to support post-surgical discharge monitoring. AMIA Annu Symp Proc 2017:2274–2278
Wagland R, Recio-Saucedo A, Simon M, Bracher M, Hunt K, Foster C, Downing A, Glaser A, Corner J (2016) Development and testing of a text-mining approach to analyse patients’ comments on their experiences of colorectal cancer care. BMJ Qual Saf 25(8):604–614. https://doi.org/10.1136/bmjqs-2015-004063
Grumett S, Snow P, Kerr D (2003) Neural networks in the prediction of survival in patients with colorectal cancer. Clin Colorectal Cancer 2(4):239–244. https://doi.org/10.3816/CCC.2003.n.005
Gao P, Zhou X, Wang ZN, Song YX, Tong LL, Xu YY, Yue ZY, Xu HM (2012) Which is a more accurate predictor in colorectal survival analysis? Nine data mining algorithms vs. the TNM staging system. PLoS ONE 7(7):e42015. https://doi.org/10.1371/journal.pone.0042015
Cowling TE, Cromwell DA, Bellot A, Sharples LD, van der Meulen J (2021) Logistic regression and machine learning predicted patient mortality from large sets of diagnosis codes comparably. J Clin Epidemiol 133:43–52. https://doi.org/10.1016/j.jclinepi.2020.12.018
Spelt L, Nilsson J, Andersson R, Andersson B (2013) Artificial neural networks–a method for prediction of survival following liver resection for colorectal cancer metastases. Eur J Surg Oncol 39(6):648–654. https://doi.org/10.1016/j.ejso.2013.02.024
Group RC-C, Arostegui I, Gonzalez N, Fernandez-de-Larrea N, Lazaro-Aramburu S, Bare M, Redondo M, Sarasqueta C, Garcia-Gutierrez S, Quintana JM (2018) Combining statistical techniques to predict postsurgical risk of 1-year mortality for patients with colon cancer. Clin Epidemiol 10:235–251. https://doi.org/10.2147/CLEP.S146729
Dimitriou N, Arandjelovic O, Harrison DJ, Caie PD (2018) A principled machine learning framework improves accuracy of stage II colorectal cancer prognosis. NPJ Digit Med 1:52. https://doi.org/10.1038/s41746-018-0057-x
Chen Z, Liu Z, Li W, Qu K, Deng X, Varma MG, Fichera A, Pigazzi A, Garcia-Aguilar J (2011) Chromosomal copy number alterations are associated with tumor response to chemoradiation in locally advanced rectal cancer. Genes Chromosomes Cancer 50(9):689–699. https://doi.org/10.1002/gcc.20891
Paredes AZ, Hyer JM, Tsilimigras DI, Moro A, Bagante F, Guglielmi A, Ruzzenente A, Alexandrescu S, Makris EA, Poultsides GA, Sasaki K, Aucejo FN, Pawlik TM (2020) A novel machine-learning approach to predict recurrence after resection of colorectal liver metastases. Ann Surg Oncol 27(13):5139–5147. https://doi.org/10.1245/s10434-020-08991-9
Lu W, Fu D, Kong X, Huang Z, Hwang M, Zhu Y, Chen L, Jiang K, Li X, Wu Y, Li J, Yuan Y, Ding K (2020) FOLFOX treatment response prediction in metastatic or recurrent colorectal cancer patients via machine learning algorithms. Cancer Med 9(4):1419–1429. https://doi.org/10.1002/cam4.2786
Xu Y, Ju L, Tong J, Zhou CM, Yang JJ (2020) Machine learning algorithms for predicting the recurrence of stage IV colorectal cancer after tumor resection. Sci Rep 10(1):2519. https://doi.org/10.1038/s41598-020-59115-y
Skrede OJ, De Raedt S, Kleppe A, Hveem TS, Liestol K, Maddison J, Askautrud HA, Pradhan M, Nesheim JA, Albregtsen F, Farstad IN, Domingo E, Church DN, Nesbakken A, Shepherd NA, Tomlinson I, Kerr R, Novelli M, Kerr DJ, Danielsen HE (2020) Deep learning for prediction of colorectal cancer outcome: a discovery and validation study. Lancet 395(10221):350–360. https://doi.org/10.1016/S0140-6736(19)32998-8
Nearchou IP, Gwyther BM, Georgiakakis ECT, Gavriel CG, Lillard K, Kajiwara Y, Ueno H, Harrison DJ, Caie PD (2020) Spatial immune profiling of the colorectal tumor microenvironment predicts good outcome in stage II patients. NPJ Digit Med 3:71. https://doi.org/10.1038/s41746-020-0275-x
Steenhuis EGM, Schoenaker IJH, de Groot JWB, Fiebrich HB, de Graaf JC, Brohet RM, van Dijk JD, van Westreenen HL, Siersema PD, de Vos Tot Nederveen Cappel WH (2020) Feasibility of volatile organic compound in breath analysis in the follow-up of colorectal cancer: a pilot study. Eur J Surg Oncol 46(11):2068–2073. https://doi.org/10.1016/j.ejso.2020.07.028
Zhao B, Gabriel RA, Vaida F, Eisenstein S, Schnickel GT, Sicklick JK, Clary BM (2020) Using machine learning to construct nomograms for patients with metastatic colon cancer. Colorectal Dis 22(8):914–922. https://doi.org/10.1111/codi.14991
Jo YY, Han J, Park HW, Jung H, Lee JD, Jung J, Cha HS, Sohn DK, Hwangbo Y (2021) Prediction of prolonged length of hospital stay after cancer surgery using machine learning on electronic health records: retrospective cross-sectional study. JMIR Med Inform 9(2):e23147. https://doi.org/10.2196/23147
Olson KA, Fleming RYD, Fox AW, Grimes AE, Mohiuddin SS, Robertson HT, Moxham J, Wolf JS Jr (2021) The enhanced recovery after surgery (ERAS) elements that most greatly impact length of stay and readmission. Am Surg 87(3):473–479. https://doi.org/10.1177/0003134820951440
Luo Z, Chen X, Zhang Y, Huang Z, Zhao H, Zhao J, Li Z, Zhou J, Liu J, Cai J, Bi X (2020) Development of a metastasis-related immune prognostic model of metastatic colorectal cancer and its usefulness to immunotherapy. Front Cell Dev Biol 8:577125. https://doi.org/10.3389/fcell.2020.577125
Vayrynen JP, Lau MC, Haruki K, Vayrynen SA, Dias Costa A, Borowsky J, Zhao M, Fujiyoshi K, Arima K, Twombly TS, Kishikawa J, Gu S, Aminmozaffari S, Shi S, Baba Y, Akimoto N, Ugai T, Da Silva A, Song M, Wu K, Chan AT, Nishihara R, Fuchs CS, Meyerhardt JA, Giannakis M, Ogino S, Nowak JA (2020) Prognostic significance of immune cell populations identified by machine learning in colorectal cancer using routine hematoxylin and eosin-stained sections. Clin Cancer Res 26(16):4326–4338. https://doi.org/10.1158/1078-0432.CCR-20-0071
Yamashita R, Long J, Longacre T, Peng L, Berry G, Martin B, Higgins J, Rubin DL, Shen J (2021) Deep learning model for the prediction of microsatellite instability in colorectal cancer: a diagnostic study. Lancet Oncol 22(1):132–141. https://doi.org/10.1016/S1470-2045(20)30535-0
Zeng Y, Chapman WC Jr, Lin Y, Li S, Mutch M, Zhu Q (2021) Diagnosing colorectal abnormalities using scattering coefficient maps acquired from optical coherence tomography. J Biophotonics 14(1):e202000276. https://doi.org/10.1002/jbio.202000276
Kang J, Choi YJ, Kim IK, Lee HS, Kim H, Baik SH, Kim NK, Lee KY (2021) LASSO-based machine learning algorithm for prediction of lymph node metastasis in T1 colorectal cancer. Cancer Res Treat 53(3):773–783. https://doi.org/10.4143/crt.2020.974
Kwak MS, Lee HH, Yang JM, Cha JM, Jeon JW, Yoon JY, Kim HI (2020) Deep convolutional neural network-based lymph node metastasis prediction for colon cancer using histopathological images. Front Oncol 10:619803. https://doi.org/10.3389/fonc.2020.619803
Herrera M, Izquierdo J, Montalvo I, Garcia-Armengol J, Roig J (2009) Identification of surgical practice patterns using evolutionary cluster analysis. Math Comput Model 50(5):705
Wang K, Feng C, Li M, Pei Q, Li Y, Zhu H, Song X, Pei H, Tan F (2020) A bibliometric analysis of 23,492 publications on rectal cancer by machine learning: basic medical research is needed. Therap Adv Gastroenterol 13:1756284820934594. https://doi.org/10.1177/1756284820934594
Dong J, Geng Y, Lu D, Li B, Tian L, Lin D, Zhang Y (2020) Clinical trials for artificial intelligence in cancer diagnosis: a cross-sectional study of registered trials in ClinicalTrialsgov. Front Oncol 10:1629. https://doi.org/10.3389/fonc.2020.01629
Brettingham-Moore KH, Duong CP, Greenawalt DM, Heriot AG, Ellul J, Dow CA, Murray WK, Hicks RJ, Tjandra J, Chao M, Bui A, Joon DL, Thomas RJ, Phillips WA (2011) Pretreatment transcriptional profiling for predicting response to neoadjuvant chemoradiotherapy in rectal adenocarcinoma. Clin Cancer Res 17(9):3039–3047. https://doi.org/10.1158/1078-0432.CCR-10-2915
Pellino G, Espin-Basany E (2021) Bowel decontamination before colonic and rectal surgery. Br J Surg 109(1):3–7. https://doi.org/10.1093/bjs/znab389
Kinross JM, Mason SE, Mylonas G, Darzi A (2020) Next-generation robotics in gastrointestinal surgery. Nat Rev Gastroenterol Hepatol 17(7):430–440. https://doi.org/10.1038/s41575-020-0290-z
Vicendese D, Marvelde LT, McNair PD, Whitfield K, English DR, Taieb SB, Hyndman RJ, Thomas R (2020) Hospital characteristics, rather than surgical volume, predict length of stay following colorectal cancer surgery. Aust N Z J Public Health 44(1):73–82. https://doi.org/10.1111/1753-6405.12932
Chen SY, Chen S, Feng W, Li Z, Luo Y, Zhu X (2021) A STING-related prognostic score predicts high-risk patients of colorectal cancer and provides insights into immunotherapy. Ann Transl Med 9(1):14. https://doi.org/10.21037/atm-20-2430
Salem H, Soria D, Lund JN, Awwad A (2021) A systematic review of the applications of expert systems (ES) and machine learning (ML) in clinical urology. BMC Med Inform Decis Mak 21(1):223. https://doi.org/10.1186/s12911-021-01585-9
Funding
This research received no specific grant from public, commercial, or not-for-profit funding agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in this review.
Ethical approval
Ethical approval is not required for this study (review).
Informed consent
Informed consent is not required for this study, as no procedures were performed by the authors in this study (review).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spinelli, A., Carrano, F.M., Laino, M.E. et al. Artificial intelligence in colorectal surgery: an AI-powered systematic review. Tech Coloproctol 27, 615–629 (2023). https://doi.org/10.1007/s10151-023-02772-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-023-02772-8