Abstract
Background
Anamorelin is a selective ghrelin receptor agonist approved for cancer cachexia in Japan. Little is known about predictors of anamorelin efficacy. This study aimed to assess the effect of diabetes on the efficacy and safety of anamorelin in patients with cancer cachexia.
Methods
Medical records of patients with advanced non-small-cell lung, gastric, pancreatic, or colorectal cancer who received anamorelin between January 2021 and March 2023 were retrospectively reviewed. The diabetic (DM) group included patients with a confirmed diagnosis of type 2 diabetes mellitus, random plasma glucose of ≥ 200 mg/dL, or hemoglobin A1c of ≥ 6.5%. The maximum body weight gain and adverse events during anamorelin administration were compared between the DM and non-DM groups. Patients with a maximum body weight gain ≥ 0 kg were classified as the responders.
Results
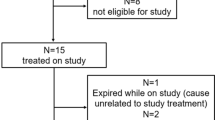
Of 103 eligible patients, 31 (30.1%) were assigned to the DM group. The DM group gained less weight (median of −0.53% vs. + 3.00%, p < 0.01) and had fewer responders (45.2% vs. 81.9%, p < 0.01) than the non-DM group. The odds ratio for non-response in the DM group was 6.55 (95% confidential interval 2.37–18.06, p < 0.01), adjusted by age and performance status. The DM group had a higher cumulative incidence of hyperglycaemic adverse events (72.2% vs. 6.3%, p < 0.01) and more discontinuations due to hyperglycaemic adverse events (25.8% vs. 4.2%, p < 0.01) than the non-DM group.
Conclusions
Patients with diabetes and cancer cachexia are less likely to gain weight with anamorelin despite a high risk of hyperglycaemic adverse events.


Similar content being viewed by others
Data availability
Data will be made available on reasonable request.
References
Fearon K, Strasser F, Anker SD et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–495
Temel JS, Abernethy AP, Currow DC et al (2016) Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol 17:519–531
Katakami N, Uchino J, Yokoyama T et al (2018) Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer 124:606–616
Hamauchi S, Furuse J, Takano T et al (2019) A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer 125:4294–4302
Iwai N, Sakai H, Oka K et al (2023) Predictors of response to anamorelin in gastrointestinal cancer patients with cachexia: a retrospective study. Support Care Cancer 31:115
Takeda T, Sasaki T, Okamoto T et al (2023) Impact of the extent of weight loss before administration on the efficacy of anamorelin in advanced pancreatic cancer patients with cachexia. Intern Med 62:1887–1893
Dev R, Bruera E, Dalal S (2018) Insulin resistance and body composition in cancer patients. Ann Oncol 29(Suppl 2):ii18–ii26
Tsilidis KK, Kasimis JC, Lopez DS et al (2015) Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 350:g7607
Hirata Y, Nomura K, Senga Y et al (2019) Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. Insight 4:e124952
Chovsepian A, Prokopchuk O, Petrova G et al (2023) Diabetes increases mortality in patients with pancreatic and colorectal cancer by promoting cachexia and its associated inflammatory status. Mol Metab 73:101729
ElSayed NA, Aleppo G, Aroda VR et al (2023) 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care 46(Suppl 1):S19–S40
Takayama H, Takiguchi T, Komura N et al (2023) Efficacy and safety of anamorelin in patients with cancer cachexia: Post-hoc subgroup analyses of a placebo-controlled study. Cancer Med 12(3):2918–2928
Garcia JM, Polvino WJ (2009) Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers. Growth Horm IGF Res 19:267–273
Miyazaki M, Sawada A, Sawamura D et al (2023) Decreased insulin-like growth factor-1 expression in response to mechanical loading is associated with skeletal muscle anabolic resistance in cancer cachexia. Growth Horm IGF Res 69–70:101536
Clemmons DR (2012) Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol Metab Clin North Am 41:425–443
Suda K, Matsumoto R, Fukuoka H et al (2016) The influence of type 2 diabetes on serum GH and IGF-I levels in hospitalized Japanese patients. Growth Horm IGF Res 29:4–10
Honors MA, Kinzig KP (2012) The role of insulin resistance in the development of muscle wasting during cancer cachexia. J Cachexia Sarcopenia Muscle 3:5–11
Martin A, Gallot YS, Freyssenet D (2023) Molecular mechanisms of cancer cachexia-related loss of skeletal muscle mass: data analysis from preclinical and clinical studies. J Cachexia Sarcopenia Muscle 14:1150–1167
Heppner KM, Tong J (2014) Mechanisms in endocrinology: regulation of glucose metabolism by the ghrelin system: multiple players and multiple actions. Eur J Endocrinol 171:R21–R32
Ohta H, Horii T, Yasu T (2023) Adverse metabolic effects on glucose in patients receiving anamorelin using a japanese claims database. Oncology 101(12):782–785
Author information
Authors and Affiliations
Contributions
KA, TN, and KM contributed to the study conceptualisation and design. KA and SH collected the data. All authors contributed to the data analysis and interpretation. KA and TN drafted the manuscript. All the other authors have critically revised and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
Tateaki Naito received grants from Otsuka Pharmaceutical K. K. and Japan’s Agency for Medical Research and Development (AMED), and personal fees from Ono Pharmaceutical Co., Ltd., and Helsinn Healthcare SA outside of the submitted work. Keita Miura received personal fees from AstraZeneca, Chugai Pharmaceutical, and Taiho Pharmaceutical outside of the submitted work. Motoki Sekikawa received personal fees from Chugai Pharmaceutical Co. Ltd. and Takeda Pharmaceutical outside of the submitted work. Hiroaki Kodama received personal fees from Chugai Pharmaceutical Co., Ltd., and Novartis Pharma K. K. outside of the submitted work. Nobuaki Mamesaya received grants and personal fees from Boehringer Ingelheim, and personal fees from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceuticals, MSD K. K., AstraZeneca K. K., and Ono Pharmaceutical Co., Ltd. outside of the submitted work. Haruki Kobayashi received personal fees from Eli Lilly K. K., Novartis Pharma K. K., Taiho Pharmaceutical., AstraZeneca K. K., Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb Company, and Daiichi Sankyo Co. outside of the submitted work. Ko Ryo received grants and personal fees from MSD K. K. and AstraZeneca K.K., and personal fees from Taiho Pharmaceuticals, Chugai Pharmaceutical Co., Ltd., Eli Lilly K. K., Ono Pharmaceutical, Daiichi Sankyo, and Takeda, outside of the submitted work. Kazushige Wakuda received grants and personal fees from Chugai Pharmaceutical Co., Ltd., AstraZeneca K.K., MSD K. K., Daiichi Sankyo Co., Ltd., and grants from Novartis Pharma K. K.; AbbVie, AMGEN, and Dizal Pharma, and personal fees from Taiho Pharmaceutical, Boehringer Ingelheim, Eli Lilly K. K., Ono Pharmaceutical, Janssen Pharmaceutical K. K., Takeda Pharmaceutical, and Nihon Kayaku outside of the submitted work. Akira Ono received personal fees from AstraZeneca K. K., Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., and Indica Laboratories outside of the submitted work. Hirotsugu Kenmotsu received personal fees from AMGEN, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical Co., Daiichi-Sankyo Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Taiho Pharmaceuticals, Takeda Pharmaceutical Co., Ltd., Merck Biopharma Co., Ltd., MSD K. K., and Pfizer, and grants and personal fees from AstraZeneca K. K., Novartis Pharma K. K., Ono Pharmaceutical Co., Ltd., and Eli Lily K. K., and grants from Loxo Oncology, outside of the submitted work. Haruyasu Murakami received grants and personal fees from AstraZeneca K. K., Takeda Pharmaceutical Co., Ltd., Daiichi-Sankyo Co., Chugai Pharmaceutical Co. Ltd., and Taiho Pharmaceutical, grants from AbbVie and IQvia, Bayer, and personal fees from Amgen, Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb Japan, MSD K. K., Pfizer Inc., Novartis Pharma K. K., Eli Lilly Japan K. K., Eisai, and Nihon Kayaku, outside of the submitted work. Toshiaki Takahashi received grants and personal fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K. K., MSD, Pfizer Japan Inc., and Amgen Inc., personal fees from Ono Pharmaceutical Co., Ltd., BMS Japan, Takeda Pharmaceuticals Co., and Novartis You, and grants from Merck Biopharma Co., Ltd., Janssen Pharmaceutical K. K., and AnHeart Therapeutics Inc. outside of the submitted work. The remaining authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ando, K., Naito, T., Hamauchi, S. et al. The efficacy and safety of anamorelin among patients with diabetes. Int J Clin Oncol (2024). https://doi.org/10.1007/s10147-024-02546-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10147-024-02546-8