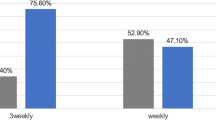
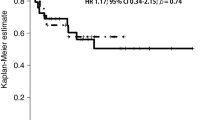
Abstract
Background
The optimal chemotherapy regimen in concurrent chemoradiotherapy (CCRT) for cisplatin-ineligible head and neck squamous cell carcinoma (HNSCC) has not been established. We aimed to evaluate the feasibility, efficacy, and safety of CCRT with weekly low-dose carboplatin for the treatment of advanced HNSCC in patients who are cisplatin-ineligible.
Methods
This prospective phase II study enrolled adult patients (age ≥ 20 years) with HNSCC receiving whole-neck irradiation including bilateral levels II–IV and who were aged (≥ 75-year-old patients with 40 mL/min estimated glomerular filtration rate [eGFR] or better) or had renal dysfunction (< 75-year-old patients with 30–60 mL/min eGFR). Carboplatin was administered weekly (area under the plasma concentration–time curve = 2.0) for up to seven cycles during concurrent radiotherapy (70 Gy/35 Fr). The primary endpoint was the completion rate of CCRT. Secondary endpoints included overall response rate and incidence of adverse events.
Results
Among the 30 patients enrolled, 28 were men. The median age was 73.5 years. Seventeen patients were < 75 years whereas 13 were ≥ 75 years old. The completion rate of CCRT was 90%. The overall response rate was 90%. Grade 3 adverse events that occurred in 10% or more patients were oral/pharyngeal mucositis (47%), leukocytopenia (20%), and neutropenia (10%). Grade 4 adverse events occurred in one patient (elevation of alanine aminotransferase level). No treatment-related deaths occurred.
Conclusion
CCRT with weekly low-dose carboplatin is a promising treatment option, with favorable feasibility, efficacy, and acceptable toxicity, for patients who are cisplatin-ineligible with advanced HNSCC.
Clinical Trial Registration Number
jRCTs031190028.


Similar content being viewed by others
Data responsibility
Y. Ueki had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Data sharing statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Chow LQM (2020) Head and neck cancer. N Engl J Med 382:60–72. https://doi.org/10.1056/NEJMra1715715
Adelstein DJ, Li Y, Adams GL et al (2003) An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 21:92–98. https://doi.org/10.1200/JCO.2003.01.008
Forastiere AA, Goepfert H, Maor M et al (2003) Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349:2091–2098. https://doi.org/10.1056/NEJMoa031317
van Hagen P, Hulshof MC, van Lanschot JJ et al (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366:2074–2084. https://doi.org/10.1056/NEJMoa1112088
Bradley JD, Paulus R, Komaki R et al (2015) Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 16:187–199. https://doi.org/10.1016/S1470-2045(14)71207-0
Ajani JA, Barthel JS, Bentrem DJ, et al (2011) Esophageal and esophagogastric junction cancers. Offic J Natl Compr Canc Netw 9:830–887 https://doi.org/10.6004/jnccn.2011.0072 (Version 5; 2022). Accessed December, 2022. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
National Comprehensive Cancer Network. Non-small cell lung cancer. Accessed December, 2022. https://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf (Version 6; 2022)
Chitapanarux I, Lorvidhaya V, Kamnerdsupaphon P et al (2007) Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer: randomised, non-inferiority, open trial. Eur J Cancer 43:1399–1406. https://doi.org/10.1016/j.ejca.2007.03.022
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Calvert AH, Newell DR, Gumbrell LA et al (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7:1748–1756. https://doi.org/10.1200/jco.1989.7.11.1748
Kataria T, Gupta D, Bisht SS et al (2015) Chemoradiation in elderly patients with head and neck cancers: a single institution experience. Am J Otolaryngol 36:117–121. https://doi.org/10.1016/j.amjoto.2014.07.015
Matsuyama H, Yamazaki K, Okabe R et al (2018) Multicenter phase I/II study of chemoradiotherapy with high-dose CDDP for head and neck squamous cell carcinoma in Japan. Auris Nasus Larynx 45:1086–1092. https://doi.org/10.1016/j.anl.2018.02.008
Suntharalingam M, Haas ML, Conley BA et al (2000) The use of carboplatin and paclitaxel with daily radiotherapy in patients with locally advanced squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys 47:49–56. https://doi.org/10.1016/s0360-3016(00)00408-9
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Sun L, Candelieri-Surette D, Anglin-Foote T et al (2022) Cetuximab-based vs carboplatin-based chemoradiotherapy for patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg 148:1022–1028. https://doi.org/10.1001/jamaoto.2022.2791
Bonner JA, Harari PM, Giralt J et al (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. New Engl J Med 354:567–578. https://doi.org/10.1056/NEJMoa053422
Wendt TG, Grabenbauer GG, Rödel CM et al (2009) Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: a randomized multicenter study. J Clin Oncol 16:1318–1324. https://doi.org/10.1200/JCO.1998.16.4.1318
Ahn MJ, D’Cruz A, Vermorken JB et al (2016) Clinical recommendations for defining platinum unsuitable head and neck cancer patient populations on chemoradiotherapy: a literature review. Oral Oncol 53:10–16. https://doi.org/10.1016/j.oraloncology.2015.11.019
Imai C, Saeki H, Yamamoto K et al (2009) Radiotherapy plus cetuximab for locally advanced squamous cell head and neck cancer in patients with cisplatin-ineligible renal dysfunction: a retrospective study. Oncol Lett 23:152. https://doi.org/10.3892/ol.2022.13271
Hamauchi S, Yokota T, Onozawa Y et al (2015) Safety and efficacy of concurrent carboplatin plus radiotherapy for locally advanced head and neck cancer patients ineligible for treatment with cisplatin. Jpn J Clin Oncol 45:1116–1121. https://doi.org/10.1093/jjco/hyv142
Kiyota N, Tahara M, Mizusawa J et al (2022) Weekly cisplatin plus radiation for postoperative head and neck cancer (JCOG1008): a multicenter, noninferiority, phase II/III randomized controlled trial. J Clin Oncol 40:1980–1990. https://doi.org/10.1200/JCO.21.01293
Acknowledgements
We thank Editage (https://www.editage.com) for its English language editing services. An abstract of this manuscript was accepted for oral presentation at the AHNS 11th International Conference on Head and Neck Cancer, which was held in July 2023 in Montreal.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conception and design: YU, KY, and AH. Data analysis and interpretation: YU, SO, YY, TT, RS, KY, KO, KS, RT, TT, YS, ST, JO, NT, and RO. Drafting of the manuscript: YU and AH. Final approval of the version to be published: All authors.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical approval statement
This trial was conducted in accordance with the Declaration of Helsinki, and all patients provided written informed consent before enrolling in the study. This trial was registered in the Japan Registry of Clinical Trials as jRCTs031190028 and approved by the Niigata University Central Review Board of Clinical Research (SP19001).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ueki, Y., Ohshima, S., Yokoyama, Y. et al. Multicenter prospective phase II trial of concurrent chemoradiotherapy with weekly low-dose carboplatin for cisplatin-ineligible patients with advanced head and neck squamous cell carcinoma. Int J Clin Oncol 29, 20–26 (2024). https://doi.org/10.1007/s10147-023-02423-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02423-w