Abstract
Background
Management of cancer-associated venous thromboembolism (VTE) is essential in cancer treatment selection and prognosis. However, currently, no method exists for assessing VTE risk associated with advanced lung cancer. Therefore, we assessed VTE risk, including driver gene mutation, in advanced lung cancer and performed a Khorana score validation.
Methods
The Rising-VTE/NEJ037 study was a multicenter prospective observational study that included patients with advanced lung cancer. In the Rising-VTE/NEJ037 study, the Khorana score was calculated for enrolled patients with available data on all Khorana score components. The modified Khorana score was based on the body mass index of ≥ 25 kg/m2, according to the Japanese obesity standard. A multivariate logistic regression analysis, including patient background characteristics, was performed to evaluate the presence of VTE 2 years after the lung cancer diagnosis.
Results
This study included 1008 patients with lung cancer, of whom 100 (9.9%) developed VTE. From the receiver operating characteristic curve analysis, VTE risk could not be determined because both the Khorana score (0.518) and modified Khorana score (0.516) showed very low areas under the curve. The risk factors for VTE in the multivariate analysis included female sex, adenocarcinoma, performance status, N factor, lymphocyte count, platelet count, prothrombin fragment 1 + 2 and diastolic blood pressure.
Conclusion
The Khorana score, which is widely used in cancer-VTE risk assessment, was less useful for Japanese patients with advanced lung cancer. Prothrombin fragment 1 + 2, a serum marker involved in coagulation, was more suitable for risk identification.
Clinical trial information
jRCTs061180025.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Venous thromboembolism (VTE) is one of the most common medical complications during cancer treatment, and its risk in patients with lung cancer is especially high [1]. Chemotherapy increases VTE risk [2, 3], and with the advancements in cancer chemotherapy, the long-term survival of patients with lung cancer is now plausible [4]. Therefore, it is increasingly important to manage complications such as VTE, and oncologists need to be mindful of its co-development when treating lung cancer patients.
The National Comprehensive Cancer Network and American Society of Clinical Oncology have published guidelines for managing cancer-associated VTE [5, 6]. Hospitalized patients admitted for purposes other than induction of short-term chemotherapy, who show no contraindications for anticoagulant therapy, should receive treatment with unfractionated heparin, low molecular weight heparin, or direct oral anticoagulants (DOACs) for preventing VTE. Furthermore, numerous risk score tools have been proposed to evaluate cancer-associated VTE [7, 8]; however, the Khorana score [7] is the most commonly used risk assessment tool for patients who are scheduled to receive chemotherapy. To prevent VTE in patients with lung cancer receiving outpatient treatments, the Khorana score should be used to evaluate VTE risk, and patients with intermediate VTE risk (Khorana scores of ≥ 2 points) should receive similar drugs as inpatients. This recommendation follows evidence of the efficacy of prophylactic administration of DOACs in two placebo-controlled trials [9, 10] conducted to determine the prophylactic effect of DOACs on VTE in patients with cancer (with Khorana scores ≥ 2 points) who were scheduled to undergo chemotherapy.
The Khorana score incorporates five clinical and laboratory risk factors: type of cancer, platelet count, hemoglobin level and use of erythropoiesis-stimulating agents, white blood cell (WBC) count, and body mass index (BMI), and lung cancer is considered to be high-risk. van Es et al. [11] evaluated the clinical utility of multiple risk scores (Khorana, PROTECHT, and CONKO scores) to predict VTE risk in a prospective cohort of 260 patients with various cancers, including lung cancer, scheduled to undergo chemotherapy. Their findings suggest that the existing risk assessment scores may be inadequate; thus, a new risk score is required. Three retrospective validation studies of the Khorana score examining only lung cancer cases have reported that the score is not very useful for assessing VTE risks [12,13,14]. A meta-analysis involving multiple cancers other than lung cancer reported that the Khorana score performance for lung cancer differed from that for other cancers and that it was not useful in predicting VTE in lung cancer [15]. Furthermore, the Khorana score criterion for obesity is a BMI of ≥ 35 kg/m2; however, validations were also performed for a modified Khorana score based on a BMI of ≥ 25 kg/m2 in an Asian population [13, 14]. Optimal management of complications such as VTE can maximize the effectiveness of highly personalized lung cancer treatments; hence, there is an urgent need to validate the Khorana score using prospective observational studies data.
The Rising-VTE/NEJ037 study, a physician-led, multicenter, prospective, observational study, determined the incidence rate of VTE and its risk factors while treating lung cancer patients for whom radical treatments were unsuitable [16]. To the best of our knowledge, the Rising-VTE/NEJ037 study is the largest prospective study involving intensive screening for VTE at the time of lung cancer diagnosis, along with a further follow-up, to assess VTE incidence. Since many cases of VTE co-developing with lung cancer are asymptomatic, an appropriate risk assessment score system is essential for identifying patients who should undergo aggressive screening and monitoring.
Here, we report the results of a validation study of the Khorana score and VTE risk, including gene mutations, such as epidermal growth factor receptor (EGFR) gene mutation status and anaplastic lymphoma kinase (ALK) fusion gene, using data from a prospective observational study of advanced lung cancer (the Rising-VTE/NEJ037 study).
Materials and methods
Patients
Case enrollment for the Rising-VTE/NEJ037 study was conducted between June 2016 and August 2018, and patients were followed up for 2 years until August 2020. The main eligibility criteria were: the diagnosis of small cell lung cancer (SCLC) or non-SCLC (NSCLC) based on cytological or histological examinations; an Eastern Co-operative Oncology Group performance status (PS) of 0–3; patients aged ≥ 20 years when consenting; SCLC for which radical surgery, radiotherapy, and chemotherapy were impossible (regardless of categorization of SCLC as a limited or extensive disease); NSCLC for which radical surgery, radiotherapy, and chemotherapy were impossible (regardless of disease stage); postoperative or disease recurrence after radical radiotherapy; patients’ conditions were contraindicated for aggressive treatments such as chemotherapy; and expected survival period of > 6 months after consent.
Since the Rising-VTE/NEJ037 study was observational, no exclusion criteria were set for case enrollment.
Patients who met the Rising-VTE/NEJ037 study eligibility criteria underwent evaluations for VTE co-development by contrast-enhanced computed tomography (CT) scans of the chest to the lower extremities or of the chest to the pelvic CT, along with lower-extremity venous ultrasound. Furthermore, patients were classified into either the observation group without VTE co-development or the cancer-associated VTE group [16]. VTE diagnoses were confirmed through a central review by two radiologists. The following evaluations of patients with deep vein thrombosis (DVT) and pulmonary thromboembolism were performed to diagnose VTE.
Validation for Khorana and modified Khorana scores
The Khorana score was calculated for the Rising-VTE/NEJ037 study enrolled patients who had available data on its components (platelet count, hemoglobin level, use of erythropoiesis-stimulating agents, WBC count and BMI) by assigning one point for each component. The modified Khorana score used a BMI of ≥ 25 kg/m2 according to the Japanese obesity standard [17]. Since all the registered Rising-VTE/NEJ037 study patients had lung cancer, one point was assigned for the primary site; therefore, the score could range from 1 to 5 points. In both the original and modified Khorana scores, the risk scores were categorized as intermediate-low risk, intermediate-high risk, and high risk for 1, 2 or ≥ 3 points, respectively.
Risk assessment for VTE
To identify VTE risk, we included patients with complete background information and clinical data, including hematological examinations and imaging data that could be used to confirm the presence of VTE. The parameters used for risk assessment included age, sex, BMI, histological classification of cancer, TNM factor, PS score, past medical history (stroke, myocardial infarction, and other malignant tumors), comorbidities (chronic obstructive pulmonary disease, rheumatoid arthritis, diabetes, hypertension, dyslipidemia and other malignant tumors), complete blood cell count, coagulation markers (d-dimer, prothrombin fragment 1 + 2 [PT F1 + 2]), liver function markers, kidney function markers, electrolyte levels, C-reactive protein (CRP) levels, brain natriuretic peptide (BNP) levels, oxygen saturation (SpO2), blood pressure, EGFR gene mutation status and ALK fusion gene. All clinical data were available during the lung cancer diagnosis.
Statistical analyses
The target sample size of the Rising-VTE/NEJ037 study was aimed to exceed those of large-scale cohorts reported so far because the incidence of VTE complication rate in Japanese patients with lung cancer was unknown at the time the study was planned. Since the prospective cohort trial at that time was on a scale of hundreds of cases, the target sample size for this trial was set to 1000.
After performing the univariate logistic regression analysis for each factor, we performed a multivariate logistic regression analysis using a stepwise method to identify VTE risk factors. Variables that were significant in the univariate analysis were employed in the multivariate model, and variables with multicollinearity problems were checked and excluded in a prior correlation analysis. To validate the Khorana and modified Khorana scores, we plotted the receiver operating characteristic (ROC) curves for each continuous variable included in the multivariate analysis and calculated their sensitivity and specificity. The ROC analysis was performed to estimate the respective cut-off values for each item to be set in the scoring process. All statistical analyses were conducted using IBM SPSS Statistics for Windows, version 24.0 (IBM Japan, Ltd., Tokyo, Japan).
Ethics
This study was conducted in accordance with the principles of the Declaration of Helsinki and the Good Clinical Practice Guidelines. The study protocol was approved by the Shimane University Institutional Review Board based on the Clinical Trials Act enacted in Japan in 2017 and published in the Japan Registry of Clinical Trials (jRCTs061180025). Written informed consent was obtained from the patients.
Results
Patient characteristics and VTE incidence rate
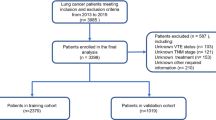
The Rising-VTE/NEJ037 study included 1021 patients diagnosed with lung cancer unsuitable for radical resection or radiation between June 2016 and August 2018 across 35 institutions in Japan. After excluding 13 patients with missing radiological data, the remaining 1008 patients constituted the full analysis set.
The enrolled patients’ median age was 70 (range 30–94) years, and most were males (714 patients, 70.8%) and had good PS (0–1, 80.6%). The most common histological lung cancer subtypes were adenocarcinoma (641 patients, 63.6%). The disease stage was assessed according to the 7th edition TNM staging system for lung cancer (UICC-7) [18]; the M1a,b stage IV disease accounted for 80% of all cases [16]. Overall, 62 and 38 patients had VTE at lung cancer diagnosis and over the two years of follow-up, respectively, totaling 100 VTE cases.
Validation of the Khorana and modified Khorana scores
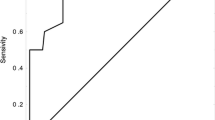
The Khorana score was calculated for 1003 patients enrolled in the Rising-VTE/NEJ037 study, and VTE co-development over the two-year observation period was confirmed in 99 patients (9.9%). According to the Khorana score, 925 (92.2%) patients were categorized as at intermediate risk of VTE development, including 730 (72.8%) and 78 (7.8%) categorized as intermediate-low (1 point) and high risk, respectively, indicating that most patients were in the intermediate risk category (Table 1A). VTE incidence rates were 9.3, 12.8 and 7.6% in the intermediate-low risk (1 point), intermediate-high risk (2 points), and high-risk categories, respectively. However, the sensitivity was low at 0.061, and ROC analysis demonstrated a very low area under the curve (AUC 0.518; 95% CI 0.458–0.578; p = 0.548), suggesting that the Khorana score could not discriminate VTE risks (Fig. 1).
According to the modified Khorana score, 907 (90.4%) patients were categorized as at intermediate risk of VTE development, including 577 (57.5%) and 96 (9.6%) categorized as intermediate-low (1 point) and high risk, respectively, indicating that most patients were in the intermediate risk category (Table 1B). VTE incidence rates were 9.2, 11.5 and 8.3% in the intermediate-low risk (1 point), intermediate-high risk (2 points), and high-risk categories, respectively. However, the sensitivity was low at 0.081 and ROC analysis demonstrated a very low AUC (0.516; 95% CI 0.457–0.575; p = 0.597), suggesting that the modified Khorana score could not discriminate VTE risks (Fig. 2).
Identification of the risk factors for VTE
After the univariate analysis (Table 2) including the patient background data (age, sex, BMI, PS, medical history, and comorbidities); tumor-related factors (histological classification, TNM factors, EGFR gene mutation and ALK fusion gene); and physiological examinations and blood biochemistry tests results (complete blood cell count, coagulation markers, liver function, kidney function, electrolytes, CRP, BNP, SpO2 and blood pressure); the multivariate analysis showed eight factors (female sex, adenocarcinoma, N3, poor PS, low lymphocyte count, low platelet count, high PT F1 + 2 and high diastolic blood pressure [DBP]) as risk factors of VTE [32].
Discussion
The Rising-VTE/NEJ037 study is the largest prospective study to intensively screen patients for VTE at lung cancer diagnosis, monitor their subsequent progression, and determine the incidence rate of VTE [16]. The rate of VTE co-development over 2 years of follow-up was 9.9%. We present the first study to use a large prospective, observational cohort data to assess VTE risk and validate the ineffectiveness of the Khorana score in patients with advanced lung cancers.
Multiple retrospective studies have reported that VTE development is common in women [19, 20]. A retrospective study [13] including Japanese patients with lung cancer suggested that VTE co-development is common in adenocarcinoma cases, and both claims are consistent with this study’s results. By contrast, while the Khorana score [9] suggests that a high platelet count increases VTE risk, our results suggest that a greater VTE risk development was associated with a low platelet count. The results of a similar prospective observational study of Japanese patients with advanced cancers [21] also indicated a low platelet count as a risk factor for VTE development. Although it has been speculated that this finding is because of the higher platelet consumption levels and subsequent tendency toward thrombus formation in the body, it is yet to be verified. The possibility that low lymphocyte counts are a risk factor for VTE was also reported for the first time in the Rising-VTE/NEJ037 study and requires future verification. Regarding the relationship between blood pressure and VTE, Mahmoodi et al. examined the risk factors for cardiovascular diseases and their association with VTE in a meta-analysis of nine clinical trials [22]. They reported that the VTE onset was inversely associated with systolic blood pressure (SBP) but not with DBP. The Rising-VTE/NEJ037 study results indicated an association between VTE incidence and elevated DBP but not with SBP. Thus, additional investigations are required to confirm whether the risk factors for cardiovascular diseases are associated with VTE.
Additionally, d-dimer was included in the Vienna VTE risk score model as a negative serum marker of VTE, and a d-dimer level of ≥ 1.44 mg/mL indicated VTE risk development [23]. Adjustment of the d-dimer cut-off level was shown to increase the Khorana score sensitivity in a prospective cohort study [24]. Here, an elevated d-dimer level was not a risk factor, and PT F1 + 2, as a serum marker involved in coagulation, was more suitable for risk identification (Table 3). d-dimer refers to the degradation products of a thrombus, a stabilized fibrin by plasmin, and it reflects the formation of fibrin in vivo only. Since PT F1 + 2 is released when activated factor X cleaves prothrombin into thrombin, its elevation indicates early-stage thrombus formation. PT F1 + 2 was reported to be particularly useful as a predictor of cancer-associated thrombosis (CAT) when used in combination with d-dimer [25], and it is necessary to reconfirm its usefulness and further consider its utilization in future studies.
With the increasing personalization of chemotherapy for lung cancer, patients are undergoing testing for gene mutations, and treatments are being tailored according to such test results [26]. Many researchers are interested in identifying the relationship between the presence or absence of gene mutations and VTE onset. A retrospective study of patients with ALK fusion gene-positive lung cancer demonstrated a significantly higher VTE incidence among 422 patients with ALK fusion gene-positive lung cancer than that in patients who were negative for the fusion gene (42.7% vs. 28.6%, p < 0.0001) [27]. Furthermore, a prospective cohort study involving 341 patients with lung cancer, including 26 positive for ALK fusion gene, indicated a higher cumulative VTE incidence rate of 26.9% in ALK fusion gene-positive patients (9.2% in ALK fusion gene-negative patients) [28]. In assessing other gene mutations (EGFR and KRAS), a meta-analysis of 25 studies suggested that neither EGFR nor KRAS gene mutations is risk factors for VTE development [29]. We only studied whether EGFR gene mutation and ALK fusion gene are risk factors for VTE and found that neither was a risk factor. This discrepancy may be due to differences in the patient populations studied. Hence, the Rising-VTE/NEJ037 study evaluated patients with advanced lung cancer, with SCLC, and with highly-differentiated squamous cell carcinoma who did not undergo genetic testing in routine clinical practice. It is necessary to conduct a prospective observational study involving only those patients positive for these gene mutations to examine the link between each gene mutation and VTE development.
This study had some limitations. First, the study was limited to Japanese patients with advanced lung cancers. Despite a 9.9% VTE incidence rate of VTE that is comparable to the reported VTE incidence rates requiring treatments in the US and Europe, many hereditary coagulation factor abnormalities associated with thrombosis show racial differences [30]. Thus, it is necessary to examine whether the results of this study are similar to those of patients in other Asian countries. Second, PT F1 + 2, which was identified as a risk factor in the present study, has not been measured in routine clinical practice or clinical trials on CAT. Therefore, it is necessary to design a new study to validate its usefulness as a risk factor. Third, the chemotherapy-induced increase in VTE risk could not be examined because > 80% of patients in the study were receiving chemotherapy. In particular, because existing data indicate an increased risk of developing VTE due to the use of angiogenesis inhibitors [31], studies to evaluate risk based on the presence or absence of chemotherapy and administered drug type will be desirable in the future.
VTE incidence during cancer treatment can be an important factor affecting patients’ vital prognosis. In routine clinical practice, a comprehensive evaluation of VTE risk development based on each patient’s clinical information and appropriate treatment selection is crucial. This is the first study to evaluate the usefulness of the Khorana and modified Khorana scores for identifying VTE development in patients with lung cancer, using data from a large-scale prospective, observational study. The findings suggest that changing the BMI in the Khorana score may not be useful for evaluating VTE risk in Japanese patients with lung cancer. From the Rising-VTE/NEJ037 dataset, we are proposing a new risk assessment scoring system for VTE development [32]. The clinical utility of a new risk assessment scoring system relevant to the pre-chemotherapy assessment of patients with advanced lung cancer needs to be confirmed in future studies. As the number of cancer patients achieving long-term survival increases, we hope that in the future, more emphasis will be placed on the diagnosis and treatment of VTE co-occurring with cancer.
References
Khorana AA, Francis CW, Culakova E et al (2007) Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 5:632–634. https://doi.org/10.1111/j.1538-7836.2007.02374.x
Moore RA, Adel N, Riedel E et al (2011) High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol 29:3466–3473. https://doi.org/10.1200/JCO.2011.35.5669
Fernandes CJ, Morinaga LTK, Alves JL et al (2019) Cancer-associated thrombosis: the when, how and why. Eur Respir Rev 28:180119. https://doi.org/10.1183/16000617.0119-2018
Takano N, Ariyasu R, Koyama J et al (2019) Improvement in the survival of patients with stage IV non-small-cell lung cancer: experience in a single institutional 1995–2017. Lung Cancer 131:69–77. https://doi.org/10.1016/j.lungcan.2019.03.008
Key NS, Khorana AA, Kuderer NM et al (2020) Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 38:496–520. https://doi.org/10.1200/JCO.19.01461
National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology: cancer-associated venous thromboembolic disease. https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Version 2.2021 (Accessed 1 Apr 2022)
Carrier M, Abou-Nassar K, Mallick R et al (2019) Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med 380:711–719. https://doi.org/10.1056/NEJMoa1814468
Khorana AA, Soff GA, Kakkar AK et al (2019) Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med 380:720–728. https://doi.org/10.1056/NEJMoa1814630
Khorana AA, Kuderer NM, Culakova E et al (2008) Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 111:4902–4907. https://doi.org/10.1182/blood-2007-10-116327
Gerotziafas GT, Taher A, Abdel-Razeq H et al (2017) A predictive score for thrombosis associated with breast, colorectal, lung, or ovarian cancer: the prospective COMPASS-cancer-associated thrombosis study. Oncologist 22:1222–1231. https://doi.org/10.1634/theoncologist.2016-0414
van Es N, Di Nisio M, Cesarman G et al (2017) Comparison of risk prediction scores for venous thromboembolism in cancer patients: a prospective cohort study. Haematologica 102:1494–1501. https://doi.org/10.3324/haematol.2017.169060
Mansfield AS, Tafur AJ, Wang CE et al (2016) Predictors of active cancer thromboembolic outcomes: validation of the Khorana score among patients with lung cancer. J Thromb Haemost 14:1773–1778. https://doi.org/10.1111/jth.13378
Hiraide M, Shiga T, Minowa Y et al (2020) Identification of risk factors for venous thromboembolism and evaluation of Khorana venous thromboembolism risk assessment in Japanese lung cancer patients. J Cardiol 75:110–114. https://doi.org/10.1016/j.jjcc.2019.06.013
Akasaka-Kihara F, Sueta D, Ishii M et al (2021) Validation of the khorana venous thromboembolism risk score in Japanese cancer patients. JACC Asia 1:259–270
van Es N, Ventresca M, Di Nisio M et al (2020) The Khorana score for prediction of venous thromboembolism in cancer patients: an individual patient data meta-analysis. J Thromb Haemost 18:1940–1951. https://doi.org/10.1111/jth.14824
Tsubata Y, Hotta T, Hamai K et al (2022) Incidence of venous thromboembolism in advanced lung cancer and efficacy and safety of direct oral anticoagulants: a multicenter, prospective, observational study (Rising-VTE/NEJ037 study). Ther Adv Med Oncol 14:1–12. https://doi.org/10.1177/17588359221110171
Japan Society for the Study of Obesity (JASSO). Obesity practice guidelines 2016. Life Science Publishing Co., Ltd. Tokyo, Japan
Goldstraw P, Crowley J, Chansky K et al (2007) The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2:706–714. https://doi.org/10.1097/JTO.0b013e31812f3c1a
Khorana AA, Francis CW, Culakova E et al (2007) Frequency, risk factors and trends for venous thromboembolism among hospitalized cancer patients. Cancer 110:2339–2346. https://doi.org/10.1002/cncr.23062
Kang MJ, Ryoo BY, Ryu MH et al (2012) Venous thromboembolism (VTE) in patients with advanced gastric cancer: an Asian experience. Eur J Cancer 48:492–500. https://doi.org/10.1016/j.ejca.2011.11.016
Kenmotsu H, Notsu A, Mori K et al (2021) Cumulative incidence of venous thromboembolism in patients with advanced cancer in prospective observational study. Cancer Med 10:895–904. https://doi.org/10.1002/cam4.3670
Mahmoodi BK, Cushman M, Anne Næss I et al (2017) Association of traditional cardiovascular risk factors with venous thromboembolism: an individual participant data meta-analysis of prospective studies. Circulation 135:7–16. https://doi.org/10.1161/CIRCULATIONAHA.116.024507
Ay C, Dunkler D, Marosi C et al (2010) Prediction of venous thromboembolism in cancer patients. Blood 116:5377–5382. https://doi.org/10.1182/blood-2010-02-270116
Li S, Gao P, Qiu J et al (2021) A modified Khorana score as a risk assessment tool for predicting venous thromboembolism in newly diagnosed advanced lung cancer. J Thromb Thrombolysis. https://doi.org/10.1007/s11239-021-02396-5
Ay C, Vormittag R, Dunkler D et al (2009) d-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol 27:4124–4129. https://doi.org/10.1200/JCO.2008.21.7752
Hanna NH, Robinson AG, Temin S et al (2021) Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol 39:1040–1091. https://doi.org/10.1200/JCO.20.03570
Al-Samkari H, Leiva O, Dagogo-Jack I et al (2020) Impact of ALK rearrangement on venous and arterial thrombotic risk in NSCLC. J Thorac Oncol 15:1497–1506. https://doi.org/10.1016/j.jtho.2020.04.033
Dou F, Zhang Y, Yi J et al (2020) Association of ALK rearrangement and risk of venous thromboembolism in patients with non-small cell lung cancer: a prospective cohort study. Thromb Res 186:36–41. https://doi.org/10.1016/j.thromres.2019.12.009
Qian X, Fu M, Zheng J et al (2021) Driver genes associated with the incidence of venous thromboembolism in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Front Oncol 11:680191. https://doi.org/10.3389/fonc.2021.680191
Miyata T, Kimura R, Kokubo Y et al (2006) Genetic risk factors for deep vein thrombosis among Japanese: importance of protein S K196E mutation. Int J Hematol 83:217–223. https://doi.org/10.1532/IJH97.A20514
Khorana AA, Dalal M, Lin J et al (2013) Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 119:648–655. https://doi.org/10.1002/cncr.27772
Tsubata Y, Hotta T, Hamai K et al (2022) A new risk-assessment tool for venous thromboembolism in advanced lung cancer: a prospective, observational study. J Hematol Oncol 15:40. https://doi.org/10.1186/s13045-022-01259-7
Acknowledgements
We thank all our patients and their families and all the site investigators for their cooperation. We also thank Dr. Hiroyuki Kuroda and Dr. Megumi Nakamura for forming the Image Assessment Committee and Dr. Takashi Yoshioka and Dr. Teruhisa Azuma for forming the Safety Monitoring Committee.
Funding
This work was funded by Daiichi Sankyo Co., Ltd. [LIX-MD-15003]. Daiichi Sankyo Co., Ltd. only provided the research funds and was not involved in protocol planning or preparation, study progress management, data collection, and statistical analysis.
Author information
Authors and Affiliations
Contributions
YT: conceptualization, methodology, investigation, data curation, writing—original draft, visualization. KH, NF, TY, RS, AN, TM, MH, SK, RH, TS and MN: investigation, writing—review & editing. KK, TH, MY, NI, KF, TK and KK: methodology, investigation, writing—review & editing. TI: conceptualization, writing—review & editing, supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Yukari Tsubata received grant and personal fees from Daiichi Sankyo Co., Ltd. and AstraZeneca K.K. and personal fees from Chugai Pharmaceuticals Inc. outside the submitted work. Naoki Furuya received personal fees from AstraZeneca K.K., Chugai Pharmaceutical, Nippon Boehringer Ingelheim Co., Ltd., Bristol-Myers Squibb Company, Eli Lilly Japan K.K., MSD K.K., Pfizer Japan Inc. Taiho Pharmaceutical, and Novartis Pharma K.K. outside the submitted work. Toshihide Yokoyama received personal fees from Eli Lilly Japan K.K., Merck Sharp & Dohme K.K., Takeda Pharmaceutical outside the submitted work. Atsushi Nakamura received grant and personal fees from AstraZeneca K.K., Thermo Fisher Scientific, Inc., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Novartis Pharma K.K., Pfizer Japan Inc., Nippon Boehringer Ingelheim Co., Ltd., and personal fees from Taiho Pharmaceutical Co., Ltd., outside the submitted work. Takeshi Masuda received personal fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Eli Lilly Japan, Nippon Boehringer Ingelheim Co., Ltd., Kyowa Kirin Co., Ltd., Ono Pharmaceutical Co., Ltd., outside the submitted work. Kazunori Fujitaka received personal fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb Company, MSD K.K., Pfizer Japan Inc., Daiichi Sankyo Co., Ltd., Eli Lilly Japan, and Nippon Boehringer Ingelheim Co., Ltd. outside the submitted work. Kunihiko Kobayashi received personal fees from AstraZeneca K.K., Takeda Pharmaceutical outside the submitted work. Takeshi Isobe received grant and personal fees from Daiichi Sankyo Co., Ltd., personal fees from AstraZeneca K.K., Pfizer Japan Inc., and Nippon Boehringer Ingelheim Co., Ltd., and grants from Pearl Therapeutics Inc. and Janssen Pharmaceutical K.K. outside the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tsubata, Y., Kawakado, K., Hamai, K. et al. Identification of risk factors for venous thromboembolism and validation of the Khorana score in patients with advanced lung cancer: based on the multicenter, prospective Rising-VTE/NEJ037 study data. Int J Clin Oncol 28, 69–78 (2023). https://doi.org/10.1007/s10147-022-02257-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02257-y