Abstract
Background
Additional histologic features of T3 colon cancer, such as tumour depth invasion beyond muscularis propria and elastic lamina invasion (ELI), have taken interest for a more accurate staging.
Methods
Patients with pT3 and pT4a (control group) colon adenocarcinoma were retrospectively collected from our institutional database. The study group was divided according to depth of tumour invasion < 5 mm and ≥ 5 mm, and into ELI − and ELI + . Chi-square test was used to compare the clinicopathological characteristics. OS and DFS were estimated using Kaplan–Meier method and compared with the log-rank test. Univariable and multivariable Cox proportional hazard models were employed to assess the effect on OS and DFS.
Results
Out of 290 pT3 tumours, 168 (58%) had a depth of tumour invasion < 5 mm and 122 (42%) ≥ 5 mm. The 5-year OS and DFS were 85.2, 68.7 and 60.9%, and 81.4, 73.9 and 60.1% in pT3 < 5 mm, pT3 ≥ 5 mm, and pT4a respectively (p = 0.001, p = 0.072). Considering ELI − (n = 157, 54%) and ELI + (n = 133, 46%), the 5-year OS and DFS were 78.9, 76.7, and 60.9%, and 75.5, 81.5, and 60.1% in ELI − , ELI + and pT4a respectively (p = 0.955, p = 0.462). At multivariable analysis, the depth of invasion was found to be an independent predictive factor for OS (HR 2.04, 95%CI 1.28–3.24, p = 0.003) and DFS (HR 1.98, 95%CI 1.24–3.18, p = 0.004), while ELI did not result a prognostic factor for OS nor DFS.
Conclusion
In pT3 colon cancer, depth of tumour invasion ≥ 5 mm is an independent risk factor for OS and DFS, whereas ELI did not result a prognostic factor affecting OS nor DFS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
TNM stage
Introduction
Colorectal cancer (CRC) is the third most common tumour diagnosed and the third cause of cancer-related deaths in the United States, accounting for 150,000 new estimated cases in 2020 [1]. To date, surgical resection of the tumour with en-bloc removal of the regional lymph nodes is the standard of care for non-metastatic colon cancer.
Pathological stage II represents the most common stage at diagnosis, and shows a variable biological behaviour and outcomes after surgery, with a 5-year overall survival (OS) ranging between 70 and 90% [2,3,4,5]. Adjuvant chemotherapy is recommended when high-risk factors are present [6,7,8]. On the contrary, stage III patients are usually treated with adjuvant chemotherapy to reduce the risk of recurrence [9]. Nevertheless, between 20 and 40% of patients develop recurrence with negative impact on survival [10, 11].
Additional prognostic factors for survival after resection were investigated [12,13,14,15]. Whereas in rectal cancer pT3 sub-classification was widely validated [16,17,18], few studies evaluated additional pathological features for a prognostic subdivision of pT3 colon cancer. Sub-classification of T3 colon cancer based on the depth of infiltration (DOI) beyond the muscularis propria, or peritoneal elastic lamina invasion (ELI) were proposed [19,20,21,22,23,24,25,26]. Yoo et al. demonstrated that a subdivision based on the measurement of the maximal DOI is correlated to nodal and distant metastasis and OS [19]. In contrast, Mrak et al. reported that subdivision based on DOI does not provide any additional information about long-term oncologic outcome [20]. Furthermore, supported by the established role of elastic lamina as landmark of visceral pleura invasion in lung cancer staging [27], several studies evaluated peritoneal ELI as a prognostic marker in CRC. Authors reported that pT3 tumours breaching the peritoneal elastic lamina (ELI +) were associated to a worse survival than pT3 tumours not breaching the peritoneal elastic lamina (ELI − ) [23,24,25,26]. On the contrary, Grin et al. found no significant differences in survival between ELI + and ELI- pT3 tumours in stage II CRC [28].
The aim of our study was to evaluate and compare the clinical significance of pT3 subdivision of intraperitoneal CRC based on the DOI beyond muscularis propria and ELI. To our knowledge, this is the first study that compares these two parameters for a prognostic sub-classification of pT3 colon cancer.
Methods
Patients selection
All patients surgically treated for primary CRC from January 1st 2008 to December 31st 2018 were collected from the prospectively maintained colorectal database of the General Surgery 3, University Hospital of Padova. Inclusion criteria were radical resection (R0) for pT3 (study group) and pT4a (control group) colorectal adenocarcinoma. Patients with extraperitoneal rectal adenocarcinoma, who underwent neoadjuvant treatment, with metastatic disease, pT1-pT2-pT4b disease, or histology other than adenocarcinoma were excluded. Patients lost on follow-up were also excluded.
Clinicopathological and treatment
For each patient data regarding age, sex, body mass index (BMI), ASA score, serum carcinoembryonic antigen (CEA), location (right/left colon), surgical approach, tumour maximal diameter, grading, lymphovascular and perineural invasion, metastatic lymph nodes, and adjuvant treatment were collected. All patients underwent standard oncological surgical resection. Follow-up was performed according to national guidelines [29]. Local recurrence was defined as recurrent disease at the site of the original CRC, whereas distant recurrence was defined as any disease identified outside the primary site.
Histopathologic analysis
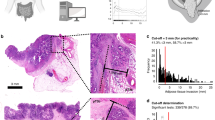
All pathological specimens were staged according to the American Joint Committee on Cancer TNM classification 8th edition [30]. According to our local gross sampling protocol, at least 4 samples from the primary tumour are analysed. All pT3 surgical specimens (n = 1160) were jointly reviewed by two gastrointestinal dedicated pathologists, who were blinded to clinical and others pathological data, to assess the sample over four most adequate to determine the DOI. Samples (n = 17) with orientation artefacts due to inadequate paraffin embedding were excluded from the selection. DOI was measured in mm from the end of longitudinal muscle layer into the nearby adipose tissue (Fig. 1). The morphometric evaluation of DOI was taken from a dedicated gastrointestinal pathologist with the support of the digital microimaging device Leica DMD108™. The presence of peritoneal ELI was jointly evaluated by two gastrointestinal pathologists, who were blinded to clinical and others pathological data, as landmark for tumour invasion between adipose tissue and serosal layer. Additional elastic stain was performed only in doubtful cases (n = 47) (Fig. 2).
Histology, 100X. Assessment of elastic lamina invasion (ELI). A and B microphotographs coloured with Hematoxylin and Eosin of adenocarcinoma glands infiltrating fatty tissue near to serosa. White arrows: adenocarcinoma glands; Red arrows: serosa. C and D microphotographs coloured with Van Gieson&Elastic fibres with elastic lamina coloured in black, C adenocarcinoma glands not infiltrating elastic lamina; D adenocarcinoma glands infiltrating elastic lamina. White arrows: adenocarcinoma glands; Red arrows: serosa; black arrows and line: elastic lamina
Statistical analysis
Considering the DOI, the study group was divided by a cut-off determined by a Receiver Operating Curve (ROC) for disease-free survival (DFS) (table of sensitivity, specificity and Youden’s J index are shown in Supplementary file, Table 1). Moreover, the study group was divided into two groups, according to the peritoneal ELI, into ELI − and ELI + .
Descriptive statistics was reported as absolute numbers and percentages, and variables distribution in the different subgroups was evaluated using the Chi-squared test. OS and DFS were evaluated using the Kaplan–Meier method and pairwise comparisons were conducted with the log-rank test. P-value associated with the pairwise comparisons underwent Benjamini–Hochberg correction to account for multiplicity of testing. The incidence of recurrence in the subgroups was evaluated using Cumulative Incidence Functions (CIF) in a competing risk framework. Differences in probabilities were evaluated using the Gray’s test. Univariable and multivariable Cox proportional hazard models were employed to assess the effect of the variables of interest on mortality and disease relapse. Results were reported as Hazard Ratio (HR), 95% confidence interval and p value. The level for statistical significance was determined at p < 0.05. All statistical analyses were performed using R software (version 4.0.3) [31].
Results
Clinicopathological characteristics
Clinicopathological characteristics are summarized in Table 1. In the study group (290 patients) there were 164(56.6%) men and 126(43.5%) women, and the median age of was 73(37–95) years. The location of the tumour was right colon in 137(47.2%) and 153(52.8%) left colon. Open surgical approach was performed in 184(63.4%) patients, while a laparoscopic approach in 106(36.6%) patients. Lymph nodes metastasis were retrieved in 121(41.8%) patients. Adjuvant chemotherapy was performed in 143(49.3%) patients. At a median follow-up of 50.5(1–192) months, local and distant recurrence occurred in 9(3.1%) and 51(17.6%) patients respectively. In the control group, 62 pT4a patients was considered for outcomes comparison.
Depth of tumour infiltration
The median value of maximum DOI of pT3 tumour infiltration was 4.0 (0.2–25) mm. The ROC curve was obtained by correlating the maximum DOI with DFS, identifying a depth of 5 mm as the best cut-off, with an area under the curve (AUC) of 0.5916 (Supplementary file, Table 1 and Fig. 1). According to this cut-off, the study cohort was divided in pT3 < 5 mm and pT3 ≥ 5 mm (Table 2). The estimated 5-year OS were 85.2, 68.8, and 60.9% in pT3 < 5 mm, pT3 > 5 mm, and pT4a respectively (log-rank test pT3 < 5 mm Vs pT3 ≥ 5 mm: p = 0.001); the estimated 5-year DFS were 81.4, 73.9, and 60.1% in pT3 < 5 mm, pT3 ≥ 5 mm, and pT4a respectively (log-rank test pT3 < 5 mm Vs pT3 > 5 mm: p = 0.07) (Fig. 3a, Fig. 3b).
Elastic lamina invasion
pT3 ELI − included 157 (54.1%) tumours and 133 (45.9%) pT3 ELI + (Table 3). The 5-years OS and DFS were 78.9, 76.7%, and 60.9%, and 75.5, 81.5, and 60.1% in ELI −, ELI + and pT4a respectively (log-rank test ELI + Vs ELI −: p = 0.9 for OS and p = 0.5 for DFS) (Fig. 3c, Fig. 3d).
Univariable and multivariable analysis
At univariable analysis, factors significantly associated with a decreased OS were age, ASA score, lymphatic invasion, lymph nodes metastasis, and DOI (pT3 ≥ 5 mm) (Table 4). Adjuvant chemotherapy resulted as protective factor (HR 0.54, 95% CI 0.34–0.86, p = 0.096). At multivariable analysis, independent risk factors were age (HR 2.93, 95% CI 1.44–5.93, p = 0.003), ASA score (HR 2.59, 95% CI 1.59–4.20, p < 0.001), lymph nodes metastasis (HR 1.78, 95% CI 1.11–2.85, p = 0.015) and DOI (HR 2.04, 95% CI 1.28–3.24, p = 0.003).
Factors significantly associated with a decreased DFS were sex, lymphatic invasion, perineural invasion, lymph nodes metastasis, and DOI (Table 4). Examined lymph nodes ≥ 12 resulted as protective factor (HR 0.50, 95%CI 0.29–0.87, p = 0.016). At multivariable analysis, independent risk factors for DFS were perineural invasion (HR 1.69, 95%CI 1.03–2.76, p = 0.034), lymph nodes metastasis (HR 2.14, 95%CI 1.20–3.80, p = 0.009), and DOI (HR 1.98, 95%CI 1.24–3.18, p = 0.004). Examined lymph nodes > 12 was confirmed as protective factor (HR 0.41, 95%CI 0.22–0.74, p = 0.004). ELI did not result associated at univariable analysis with OS (HR 0.98, 95%CI 0.62–1.55, p = 0.963) nor DFS (HR 0.93, 95%CI 0.93–1.22, p = 0.776). Association between DOI and ELI, and lymph nodes metastasis was also examined using a univariable logistic regression approach. Both pT3 ≥ 5 mm and ELI + were factors related to lymph nodes metastasis (OR 1.87, 95%CI 1.61–3.02, p = 0.010, OR 1.928, 95%CI 1.197–3.105, p = 0.007 respectively).
Sub-analysis according to N status
Considering only N0 patients (169 patients), the 5-year OS was respectively 86.8, and 85.6% in pT3 < 5 mm and pT3 ≥ 5 mm, and the 5-year DFS were 83.4, and 88.6% (log-rank test p = 0.72 and p = 0.52) (Supplementary file, Fig. 2). Estimated 5-year recurrence rate was 15.9 and 10.8% in pT3 < 5 mm, pT3 ≥ 5 mm respectively (Grey’s test p = 0.48) (Supplementary file, Fig. 3). At univariable analysis, DOI did not result associated with an impaired OS nor DFS (HR 1.130, 95%CI 0.582–2.191, p = 0.719; HR 0.731, 95%CI 0.281–1.903, p = 0.521) (Supplementary file, Table 2 and Table 3).
Considering only N + patients (121 patients), the 5-year OS was respectively 82.1 and 50% in pT3 < 5 mm and pT3 ≥ 5 mm, and the 5-year DFS was 77.7 and 59.3% (log-rank test p < 0001 and p = 0.02) (Fig. 4). Estimated 5-year recurrence rate was 22.0 and 39.4%, 41.4 and 21.9% in pT3 < 5 mm, pT3 ≥ 5 mm, ELI − and ELI + respectively (Grey’s test p = 0.023 and p = 0.069 respectively) (Supplementary file, Fig. 5). At multivariable analysis, DOI pT3 ≥ 5 mm resulted associated with an impaired OS and DFS (HR 3.26, 95% CI 1.564–6.77, p = 0.002) (HR 2.309, 95% CI 1.147–4.645, p = 0.019), while at univariable analysis ELI did not result a predictive factor for OS and DFS (HR 0.720, 95% CI 0.387–1.340, p = 0.521, HR 0.547, 95% CI 0.278–1.076, p = 0.081) (Table 5).
Discussion
Assessment of additional pathological features might allow an accurate risk stratification to identify high-risk stage II CRC patients who benefit from adjuvant therapy. A pT3 sub-classification based on DOI was proposed by several authors, while others strongly suggested a sub-classification based on peritoneal ELI.
We used a subdivision of pT3 using a cut-off, determined by using a ROC for DFS, of 5 mm DOI beyond the muscular layer, which resulted to affect survival. A DOI > 15 mm was proposed by Merkel et al. as a major risk factors for stage II colon carcinoma [32], while various cut-offs between 2 and 10 mm were demonstrated for the subdivision of T3 CRC [16, 33]. Yoo et al., similarly to Pollheimer et al., divided pT3 patients using 4 different cut-offs (< 1 mm; 1–5 mm; 5–15 mm; > 15 mm), reporting 5 mm cut-off as the strongest prognostic factor in both their analysis [19, 34]. Recently, Nomura et al. reported 148 pT3 CRC subdivided into T3a (< 1 mm); T3b (1–5 mm); T3c (> 5 mm) and confirmed a 5 mm as optimal cut-off predictor for recurrence [21].
At univariable and multivariable analysis pT3 ≥ 5 mm category resulted as independent prognostic factor both for OS and DFS. Similarly, Bori et al. divided 593 CRC by a 5 mm cut-off, reporting that pT3 < 5 mm is associated with an improved long-term outcomes in terms of nodal involvement and distant metastasis [35]. Furthermore, similar results were reported by several authors [19, 21, 36], in particular Akagi et al. reported that pT3 ≥ 5 mm was the strongest independent risk factor for recurrence on 202 cases of stage II colon cancer, and proposed adjuvant chemotherapy as indicated for these patients [37]. Based on this rationale, our sub-analysis on pT3N0 and pT3N + aimed to determine if the DOI ≥ 5 mm may be a recommendation for adjuvant treatment. In this setting, the DOI failed to result as predictive factors for survival in pT3N0 patients, although it was confirmed as a strong independent predictor of OS and DFS in pT3N + patients (HR 3.26, 95%CI 1.56–6.78, p = 0.002, and HR 2.31 95%CI 1.15–4.65, p = 0.019 respectively). In this setting, DOI confirmed to be an index of advanced disease in pT3N + patients, as far as Kaplan–Meier survival analysis showed that in N + group pT3 ≥ 5 mm patients had a survival even worse than pT4 control group (5-year OS 50.0 Vs 60.9%, 5-year DFS 59.3 Vs 60.1% respectively). For this reason, we also strongly suggest to consider DOI as a high-risk factor, that may help in case of doubt for adjuvant treatment, even in pT3N0 patients.
Alternatively to the measurement of DOI, Shinto et al. used the peritoneal ELI as a cut-off, finding an increased recurrence rate and decrease of survival for tumours with ELI [24]. Kojima et al. reported that ELI had a strong impact on survival and may be useful as a pathologic diagnostic tool to predict high-risk colon cancer [23]. In contrast, Grin et al. observed no significant differences in DFS between 186 patients with pT3 ELI- and ELI + stage II CRC [28]. In particular, they underlined the limitation of ELI as predictive factor, since in 18.3% of the cases the elastic lamina was not identifiable elastic lamina, mostly in right-sided tumours, despite repeated staining and assessment of multiple blocks. Even when identifiable, the ELI assessment was often challenging, because of severe distortion or destruction caused by a fibroinflammatory reaction close to the tumour [22]. To note, in our analysis ELI resulted recognizable in every patient during pathological revision and elastic stain were performed only in doubtful cases. To overcome this limitation, Liang et al. and Nakanishi et al. sustained the routine use of elastic stain as a useful and inexpensive method to demonstrate peritoneal ELI by tumour that should be considered for routine use in all CRC [26, 38]. Lu et al., proposed ELI assessed with elastic stain as prognostic factors for stage II colon cancer, and might be an indication to postoperative adjuvant chemotherapy [39]. Recently, a meta-analysis including six studies, recommended the sub-categorization of pT3 CRC by ELI for better prognostic assessment and treatment strategy of patients with CRC [40]. Our analysis failed to confirm any significant impact of ELI on oncological outcome, and ELI as predictive factor at univariable analysis. To note, lymph nodes metastasis and adjuvant treatment have a main role in long-term outcome, and the prognostic role of ELI may have been mitigated by the high rate of Stage III who underwent adjuvant treatment (70% in ELI + in pT3N + patients). As reported by Yokota et al., even if ELI resulted as independent risk factor for RFS and OS, RFS resulted almost identical when comparing pT3N + /ELI + to pT3N + /ELI- in patients with no adjuvant treatment [25].
Along with DOI and ELI, the circumferential resection margin (CRM) was investigated in colon cancer also, whereas its prognostic role in rectal cancer is well known. NCDB registry showed that a positive CRM were common in pT4 (26–32% of patients), whereas in pT2 and pT3 colon cancer is less common (6 and 11% of patients respectively) [41]. A positive CRM should be an indication for adjuvant treatment in stage II colon cancer, however in only 9% of these patients were reported [42]. Nevertheless, the histopathological parameters that we considered are more common in T3 colon cancer patients, representing in both cases more than 40% of the patients. Furthermore, beside pathological analysis in the last years circulating tumour DNA (ctDNA) is assuming an increasing role in the prognosis of colon cancer. Our group previously reported that an increased level of ctDNA was associated to a poor prognosis [43], whereas an Australian multicenter RCT reported ctDNA as independent predictors of recurrence-free survival [44]. Unfortunately, even considering these promising data, the ctDNA is not commonly tested in the normal follow-up of colon cancer, whereas the evaluation of DOI and ELI are an unexpensive extension of normal pathological examinations.
There are some limitations in this study. First, this is a single-institution retrospective study. Even if we considered the limitation of a single institution study, in our study group almost 300 colon cancer were re-evaluated by dedicated colorectal pathologists. Second, the patients included in the study covered a wide timespan of 10 years, during which several changes occurred in staging modalities, preoperative treatment, anaesthesiological, pathological, and surgical techniques, follow-up and adjuvant treatment. All these changes may have a potential impact on OS and DFS. Lastly, we did not identify other confounding factors, such as high-risk features in Stage II or Stage III not treated with adjuvant therapy. In our cohort high-risk stage II and stage III patients routinely underwent adjuvant treatment. Patients in these stages who did not receive any adjuvant treatment might have been unfit for further treatment, and this may have an adverse impact on survival. For these reasons, the results of a further subgroup analysis by considering these confounding factors may not be reliable also.
Conclusion
In our study, the DOI beyond the muscular layer of colonic wall, using a cut-off of 5 mm, is an independent risk factor both for OS and DFS, and may be considered as high-risk feature in pT3 colon cancer. ELI was not resulted to be a prognostic factor affecting OS and DFS. To our knowledge, this is the first study that compares these 2 parameters for a prognostic sub-classification of pT3 colon cancer.
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2000. CA Cancer J Clin 70(1):7–30. https://doi.org/10.3322/caac.21590
Morris M, Platell C, McCaul K et al (2007) Survival rates for stage II colon cancer patients treated with or without chemotherapy in a population-based setting. Int J Colorectal Dis 22(8):887–895. https://doi.org/10.1007/s00384-006-0262-y
Böckelman C, Engelmann BE, Kaprio T et al (2015) Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol 54(1):5–16. https://doi.org/10.3109/0284186x.2014.975839
Gertler R, Rosenberg R, Schuster T et al (2009) Defining a high-risk subgroup with colon cancer stages I and II for possible adjuvant therapy. Eur J Cancer 45(17):2992–2999. https://doi.org/10.1016/j.ejca.2009.07.008
Tsikitis VL, Larson DW, Huebner M et al (2014) Predictors of recurrence free survival for patients with stage II and III colon cancer. BMC Cancer 14:336. https://doi.org/10.1186/1471-2407-14-336
Kannarkatt J, Joseph J, Kurniali PC et al (2017) Adjuvant chemotherapy for stage II colon cancer: a clinical dilemma. J Oncol Pract 13(4):233–241. https://doi.org/10.1200/jop.2016.017210
Lee JJ, Chu E (2017) Adjuvant chemotherapy for stage II colon cancer: the debate goes on. J Oncol Pract 13(4):245–246. https://doi.org/10.1200/jop.2017.022178
Fang SH, Efron JE, Berho ME et al (2014) Dilemma of stage II colon cancer and decision making for adjuvant chemotherapy. J Am Coll Surg 219(5):1056–1069. https://doi.org/10.1016/j.jamcollsurg.2014.09.010
Taieb J, Tabernero J, Mini E et al (2014) Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol 15(8):862–873. https://doi.org/10.1016/s1470-2045(14)70227-x
André T, Boni C, Navarro M et al (2009) Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 27(19):3109–3116
Wilkinson NW, Yothers G, Lopa S et al (2010) Long-term survival results of surgery alone versus surgery plus 5-fluorouracil and leucovorin for stage II and stage III colon cancer: pooled analysis of NSABP C-01 through C-05 A baseline from which to compare modern adjuvant trials. Ann Surg Oncol 17(4):959–966. https://doi.org/10.1245/s10434-009-0881-y
Mayanagi S, Kashiwabara K, Honda M et al (2018) Risk factors for peritoneal recurrence in stage II to III colon cancer. Dis Colon Rectum 61(7):803–808. https://doi.org/10.1097/dcr.0000000000001002
Osterman E, Glimelius B (2018) Recurrence risk after up-to-date colon cancer staging, surgery, and pathology: analysis of the entire swedish population. Dis Colon Rectum 61(9):1016–1025. https://doi.org/10.1097/dcr.0000000000001158
Guraya SY (2019) Pattern, stage, and time of recurrent colorectal cancer after curative surgery. Clin Colorectal Cancer 18(2):e223–e228. https://doi.org/10.1016/j.clcc.2019.01.003
Wang L, Hirano Y, Ishii T et al (2020) Left colon as a novel high-risk factor for postoperative recurrence of stage II colon cancer. World J Surg Oncol 18(1):54. https://doi.org/10.1186/s12957-020-01818-7
Willett CG, Badizadegan K, Ancukiewicz M et al (1999) Prognostic factors in stage T3N0 rectal cancer: do all patients require postoperative pelvic irradiation and chemotherapy? Dis Colon Rectum 42(2):167–173. https://doi.org/10.1007/bf02237122
Harewood GC, Kumar KS, Clain JE et al (2004) Clinical implications of quantification of mesorectal tumor invasion by endoscopic ultrasound: all T3 rectal cancers are not equal. J Gastroenterol Hepatol 19(7):750–755. https://doi.org/10.1111/j.1440-1746.2004.03356.x
Miyoshi M, Ueno H, Hashiguchi Y et al (2006) Extent of mesorectal tumor invasion as a prognostic factor after curative surgery for T3 rectal cancer patients. Ann Surg 243(4):492–498. https://doi.org/10.1097/01.sla.0000205627.05769.08
Yoo HY, Shin R, Ha HK et al (2012) Does t3 subdivision correlate with nodal or distant metastasis in colorectal cancer? J Korean Soc Coloproctol 28(3):160–164. https://doi.org/10.3393/jksc.2012.28.3.160
Mrak K, Jagoditsch M, Leibl S et al (2011) Influence of pT3 subgroups on outcome of R0-resected colorectal tumors. South Med J 104(11):722–730. https://doi.org/10.1097/SMJ.0b013e3182321271
Nomura M, Takahashi H, Fujii M et al (2019) Clinical significance of invasion distance relative to prognosis in pathological T3 colorectal cancer. Oncol Lett 18(5):5614–5620. https://doi.org/10.3892/ol.2019.10913
Kojima M, Yokota M, Saito N et al (2012) Elastic laminal invasion in colon cancer: diagnostic utility and histological features. Front Oncol 2:179
Kojima M, Nakajima K, Ishii G et al (2010) Peritoneal elastic laminal invasion of colorectal cancer: the diagnostic utility and clinicopathologic relationship. Am J Surg Pathol 34(9):1351–1360. https://doi.org/10.1097/PAS.0b013e3181ecfe98
Shinto E, Ueno H, Hashiguchi Y et al (2004) The subserosal elastic lamina: an anatomic landmark for stratifying pT3 colorectal cancer. Dis Colon Rectum 47(4):467–473. https://doi.org/10.1007/s10350-003-0083-9
Yokota M, Kojima M, Nomura S et al (2014) Clinical impact of elastic laminal invasion in colon cancer: elastic laminal invasion-positive stage II colon cancer is a high-risk equivalent to stage III. Dis Colon Rectum 57(7):830–838
Nakanishi Y, LeVea C, Dibaj S et al (2016) Reappraisal of serosal invasion in patients with T3 colorectal cancer by elastic stain: clinicopathologic study of 139 surgical cases with special reference to peritoneal elastic lamina invasion. Arch Pathol Lab Med 140(1):81–85
Chen T, Luo J, Wang R et al (2017) Visceral pleural invasion predict a poor survival among lung adenocarcinoma patients with tumor size ≤ 3cm. Oncotarget 8(39):66576–66583. https://doi.org/10.18632/oncotarget.16476
Grin A, Messenger DE, Cook M et al (2013) Peritoneal elastic lamina invasion: limitations in its use as a prognostic marker in stage II colorectal cancer. Hum Pathol 44(12):2696–2705. https://doi.org/10.1016/j.humpath.2013.07.013
AIOM (2019) Linee guida NEOPLASIE DEL RETTO E ANO. https://www.aiom.it/wp-content/uploads/2019/10/2019_LG_AIOM_Retto_ano.pdf. Accessed 22/10/2020
Amin MB, Edge SB, Greene FL et al (2017) AJCC cancer staging manual. Springer, New York
R Development Core Team (2017) R: a language and environment for statistical computing. R Development Core Team, Vienna
Merkel S, Wein A, Günther K et al (2001) High-risk groups of patients with stage II colon carcinoma. Cancer 92(6):1435–1443
Burdy G, Panis Y, Alves A et al (2001) Identifying patients with T3–T4 node-negative colon cancer at high risk of recurrence. Dis Colon Rectum 44(11):1682–1688
Pollheimer MJ, Kornprat P, Pollheimer VS et al (2010) Clinical significance of pT sub-classification in surgical pathology of colorectal cancer. Int J Colorectal Dis 25(2):187–196
Bori R, Sejben I, Svébis M et al (2009) Heterogeneity of pT3 colorectal carcinomas according to the depth of invasion. Pathol Oncol Res 15(3):527
Wong SKC, Jalaludin BB, Henderson CJ et al (2008) Direct tumor invasion in colon cancer: correlation with tumor spread and survival. Dis Colon Rectum 51(9):1331–1338
Akagi Y, Shirouzu K, Kinugasa T (2013) Extramural extension as indicator for postoperative adjuvant chemotherapy in stage IIA (pT3N0) colon cancer. J Surg Oncol 108(6):358–363
Liang W-Y, Chang W-C, Hsu C-Y et al (2013) Retrospective evaluation of elastic stain in the assessment of serosal invasion of pT3N0 colorectal cancers. Am J Surg Pathol 37(10):1565–1570
Lu J, Hu X, Meng Y et al (2018) The prognosis significance and application value of peritoneal elastic lamina invasion in colon cancer. PLoS ONE 13(4):e0194804
Odate T, Vuong HG, Mochizuki K et al (2019) Assessment of peritoneal elastic laminal invasion improves survival stratification of pT3 and pT4a colorectal cancer: a meta-analysis. J Clin Pathol 72(11):736–740
Healy MA, Peacock O, Hu CY et al (2020) High rate of positive circumferential resection margin in colon cancer: a national appraisal and call for action. Ann Surg. https://doi.org/10.1097/sla.0000000000004682
Goffredo P, Zhou P, Ginader T et al (2020) Positive circumferential resection margins following locally advanced colon cancer surgery: risk factors and survival impact. J Surg Oncol 121(3):538–546. https://doi.org/10.1002/jso.25801
Bedin C, Enzo MV, Del Bianco P et al (2017) Diagnostic and prognostic role of cell-free DNA testing for colorectal cancer patients. Int J Cancer 140(8):1888–1898. https://doi.org/10.1002/ijc.30565
Tie J, Cohen JD, Wang Y et al (2019) Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol 5(12):1710–1717. https://doi.org/10.1001/jamaoncol.2019.3616
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement. No external funding for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have indicated that they have no conflict of interest to declare. Matteo Fassan received research funding from Astellas Pharma, Macrophage Pharma, and QED Therapeutics and had roles as consultant or advisor for Astellas Pharma, GSK-Tesaro, MSD, Roche, and Astra Zeneca. The other authors have no conflicts of interest to declare regarding the present manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Macchi, L., Bao, Q.R., Albertoni, L. et al. Prognostic significance of additional histologic features for subclassification of pathological T3 colon cancer. Int J Clin Oncol 27, 1428–1438 (2022). https://doi.org/10.1007/s10147-022-02192-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02192-y