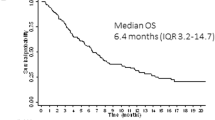
Abstract
Background
Until recently, no effective targeted therapies for FLT3-mutated (FLT3mut+) relapsed/refractory (R/R) acute myeloid leukemia (AML) were available in Japan. The FLT3 inhibitor, gilteritinib, was approved in Japan for patients with FLT3mut+ R/R AML based on the phase 3 ADMIRAL trial, which demonstrated the superiority of gilteritinib over salvage chemotherapy (SC) with respect to overall survival (OS; median OS, 9.3 vs 5.6 months, respectively; hazard ratio, 0.64 [95% confidence interval 0.49, 0.83]; P < 0.001).
Methods
We evaluated the Japanese subgroup (n = 48) of the ADMIRAL trial, which included 33 patients randomized to 120-mg/day gilteritinib and 15 randomized to SC.
Results
Median OS was 14.3 months in the gilteritinib arm and 9.6 months in the SC arm. The complete remission/complete remission with partial hematologic recovery rate was higher in the gilteritinib arm (48.5%) than in the SC arm (13.3%). After adjustment for drug exposure, fewer adverse events (AEs) occurred in the gilteritinib arm than in the SC arm. Common grade ≥ 3 AEs related to gilteritinib were febrile neutropenia (36%), decreased platelet count (27%), and anemia (24%).
Conclusion
Findings in Japanese patients are consistent with those of the overall ADMIRAL study population.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) is the most common leukemia among Japanese adults, and accounts for approximately 70% of all myeloid leukemias [1, 2]. Patients with relapsed AML have a poor prognosis, with a 5-year survival rate of 10–11% [3, 4]. Activating mutations in fms-like tyrosine kinase 3 (FLT3) occur in approximately 30% of patients with AML [5, 6], primarily as internal tandem duplication (FLT3-ITD) mutations in the juxtamembrane region (~ 20–25%) and missense point mutations in the tyrosine kinase domain (FLT3-TKD; ~ 5–7%) [5, 7,8,9,10,11]. In AML, FLT3-ITD mutations confer a strong negative impact on survival[12,13,14]; FLT3-TKD point mutations have been implicated in resistance to FLT3 inhibitors [15, 16].
Several FLT3 tyrosine kinase inhibitors (TKIs) are under development or have been approved for the treatment of FLT3-mutated (FLT3mut+) AML. First-generation multi-targeted FLT3 TKIs (sunitinib, sorafenib, midostaurin, lestaurtinib, ponatinib) [17] lacked specificity for FLT3-ITD, resulting in short-lived antileukemic activity, especially as single agents [18,19,20,21]. Second-generation FLT3 TKIs (gilteritinib and quizartinib) boasting higher potency and greater specificity for FLT3 were subsequently developed [17]. While quizartinib demonstrated single-agent activity in relapsed/refractory (R/R) FLT3-ITD–positive (FLT3-ITD +) AML patients [22], treatment-emergent FLT3-TKD mutations leading to drug resistance [15] and myelosuppression due to quizartinib’s effects on c-kit [23] are of concern.
Gilteritinib is a highly selective, oral FLT3 inhibitor with activity against both FLT3-ITD and -TKD mutations with only weak activity against c-Kit [24, 25]. The phase 1/2 CHRYSALIS study demonstrated that single-agent gilteritinib had a favorable safety profile and, at ≥ 80-mg/day doses, induced ≥ 90% inhibition of FLT3 autophosphorylation with a composite complete remission (CRc) rate of 41% in patients with R/R FLT3mut+ AML [26]. A starting dose of 120 mg/day was recommended for further study [26].
Gilteritinib was the first FLT3 TKI approved by the Ministry of Health Labor and Wealth (MHLW) in Japan for patients with R/R FLT3mut+ AML [27]. Approval was based on interim results from the phase 3 ADMIRAL study (NCT02421939) of gilteritinib versus salvage chemotherapy (SC) in patients with R/R FLT3mut+ AML, where 40 of 142 (28%) patients in the gilteritinib arm had achieved complete remission (CR) or CR with partial hematologic recovery (CRh) [27]. Final results from ADMIRAL demonstrated significantly longer overall survival (OS) with gilteritinib than with SC (9.3 vs 5.6 months, respectively; hazard ratio [HR] for death, 0.64; 95% confidence interval [CI] 0.49, 0.83; P < 0.001) [28].
As treatment efficacy and safety may vary by ethnicity, evaluation of gilteritinib in Japanese patients with R/R FLT3mut+ AML is warranted. The safety and antitumor effects of gilteritinib were reported in a phase 1 study of 24 Japanese patients with R/R AML; however, only 3 FLT3mut+ Japanese patients had received ≥ 80-mg gilteritinib [29]. The ADMIRAL trial included 54 R/R FLT3mut+ AML patients from Asian countries, with most (89%; 48 of 54) being Japanese. A subgroup analysis was conducted to confirm the clinical risk–benefit profile of gilteritinib in Japanese patients enrolled in the ADMIRAL trial.
Materials and methods
Trial design and oversight
This randomized phase 3, open-label study (NCT02421939; ADMIRAL) was conducted at 107 centers in 14 countries [28]. Japanese patients were enrolled at 20 sites across Japan. The trial was reviewed and approved by site-specific institutional review boards or ethics committees and conducted according to the principles of the Declaration of Helsinki. All patients provided written informed consent at enrollment. All authors had access to study data and have ascertained its accuracy and adherence to the study protocol.
Patients
Complete details of the ADMIRAL population are provided in the primary publication [28]. Adults with AML were eligible if they were refractory to one or two cycles of conventional anthracycline-containing induction therapy or were experiencing hematologic relapse after first CR. Patients ineligible for anthracycline-containing induction regimens could participate if they had completed ≥ 1 cycle of alternative standard therapy judged as the optimum choice to induce remission. At enrollment, patients’ marrow and blood were screened for FLT3 mutations by a central laboratory. Enrollment based on local FLT3 mutation testing was permitted in patients with rapidly proliferative disease. Patients were required to have either FLT3-ITD or FLT3-TKD D835 or I836 mutations (Invivoscribe; San Diego, CA) using a polymerase chain reaction-based assay modeled on published methods (LeukoStrat® CDx) [30].
Randomization and treatments
A brief description of the ADMIRAL study design, randomization, and treatment are described (Supplement, Figure S1), with further details in the primary publication [28]. Briefly, enrolled patients were randomized 2:1 to receive 28-day cycles of once-daily gilteritinib (120 mg) or preselected high- or low-intensity SC. High- or low-intensity SC regimens were selected by study investigators before randomization from the following options: high-intensity SC: mitoxantrone, etoposide, and cytarabine (MEC)[31], or fludarabine, cytarabine, granulocyte-colony stimulating factor, and idarubicin (FLAG-IDA)[32]; or low-intensity SC: low-dose cytarabine (LoDAC) or azacitidine (AZA). High-intensity chemotherapy was administered for one to two cycles; gilteritinib or low-intensity SC were administered until documented lack of clinical benefit, intolerance, or other protocol-defined discontinuation criterion [28].
Endpoints and assessments
The co-primary endpoints were OS and the rates of CR/CRh, where CRh was defined as CR with a platelet count of > 0.5 Gi/L and an absolute neutrophil count of > 0.5 Gi/L. Key secondary endpoints were event-free survival (EFS) and CR rate, where EFS was defined as the time from the date of randomization until the date of documented relapse (excludes relapse after partial remission), treatment failure, or death. The CR/CRh rate was evaluated in the first interim analysis in the gilteritinib arm only and was also summarized in the final analysis for both treatment arms. Overall survival, EFS, CR rate, and other response outcomes were evaluated in the final analysis. Treatment response was assessed using the modified International Working Group criteria [33]; best response was captured at any postbaseline visit. Treatment-emergent adverse events (TEAEs) were graded according to the Common Terminology Criteria for Adverse Events (v4.03). The exposure-adjusted incidence of TEAEs per patient-year (PY) was calculated by dividing exposure duration in years by the number of patients in each treatment arm. Postbaseline (i.e., 29 days after first dose until the last dose) transfusion status was evaluated in patients on gilteritinib therapy for ≥ 84 days; transfusion independence was achieved if no red blood cell/platelet transfusions were administered for 56 consecutive days during the postbaseline period. The incidence and duration of hospitalization were also evaluated.
Statistical analysis
Complete details on statistical analyses in the ADMIRAL trial have been previously published [28]. The CR/CRh rate in the gilteritinib arm was assessed at the first planned interim analysis when approximately 141 patients in the intention-to-treat (ITT) population had reached 112 days (four treatment cycles) after randomization or the first dose; interim evaluation of CR/CRh rate had no impact on trial conduct [28].
The Japanese subgroup was analyzed similarly to the overall ITT population. The Kaplan–Meier method combined with the Greenwood formula was used to determine OS and EFS and corresponding 95% CIs. As the trial was designed to test hypotheses in the overall ITT population, it was not powered to detect cross-arm differences in this subgroup analysis.
Results
Patient disposition
Of the 625 patients screened from October 2015 to February 2018, 371 (ITT population) were randomized to gilteritinib (n = 247) or SC (n = 124), which included 48 Japanese patients (gilteritinib, n = 33; SC, n = 15) (Fig. 1). At the time of primary analysis (September 17, 2018), 24 of 48 (50%) Japanese patients remained alive (n = 15/33, gilteritinib; n = 9/15, SC) and 7 remained on gilteritinib therapy. In the Japanese subgroup, 79% (n = 11/14) of patients in the SC arm received low-intensity chemotherapy compared with 38% (n = 41/109) of SC-treated patients in the overall safety analysis set (SAF) population.
Patient disposition: Japanese ITT population. AML acute myeloid leukemia, FLAG-IDA fludarabine, cytarabine, and idarubicin plus granulocyte colony-stimulating factor, ITT intention-to-treat, LoDAC low-dose cytarabine, MEC mitoxantrone, etoposide, and cytarabine, mut + mutated, R/R relapsed or refractory
Demographic and baseline characteristics
As shown in Table 1, 46% (n = 22/48) of patients had relapsed AML (gilteritinib, 52% [n = 17/33]; SC, 33% [n = 5/15]) and 54% (n = 26/48) had primary refractory disease (gilteritinib, 48% [n = 16/33]; SC, 67% [n = 10/15]). The proportion of Japanese patients aged ≥ 65 years was higher in the SC arm (n = 13/15; 87%) than in the gilteritinib arm (n = 15/33; 45%). Japanese patients had lower body weight (mean ± standard deviation [SD]: 52.91 ± 11.10 kg) compared with the overall ITT population (mean ± SD: 71.82 ± 20.25 kg).
Study drug exposure and dose modifications
The median duration of exposure to gilteritinib in the Japanese subgroup was 5.1 months
(154 days) (Table 2), which was longer than that reported for the overall SAF population (4.1 months); the median duration of exposure to SC was the same in both populations (0.9 months). Overall, 33% of gilteritinib-treated patients (n = 11/33) in the Japanese subgroup had dose increases to 200 mg/day (Table 2), which was similar to the overall SAF population (32%). Gilteritinib dose reductions were slightly higher in the Japanese subgroup (39%; n = 13/33) than in the overall SAF population (31%); dose interruptions were also higher in the Japanese subgroup (67% vs 50%, respectively).
Survival outcomes
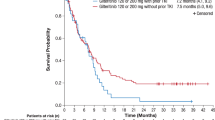
In the overall ITT population, median OS was 9.3 months in the gilteritinib arm and 5.6 months in the SC arm; 1-year survival rates were 37% for patients who received gilteritinib compared with 17% for patients who received SC. In the Japanese subgroup, the median OS was 14.3 months in gilteritinib-treated patients and 9.6 months in patients treated with SC (Fig. 2); 1-year survival rates were 54.3% in the gilteritinib arm versus 26.3% in the SC arm.
Response outcomes
In the overall ITT population, the rate of CR/CRh was higher in the gilteritinib arm than in the chemotherapy arm (34.0% vs 15.3%, respectively), as was the CR rate (21.1% vs 10.5%, respectively). Similarly, CR/CRh rates in the Japanese subgroup were also higher in the gilteritinib arm (48.5%) than in the SC arm (13.3%) (Table 3). Rates of CR/CRh before HSCT in the Japanese subgroup gilteritinib and SC arms were 30.3% and 13.3%, respectively, and were comparable to the overall ITT population (gilteritinib, 26.3%; SC, 15.3%); CR rates (gilteritinib, 24.2%; SC, 6.7%) in the Japanese subgroup were also similar to the overall ITT population (gilteritinib, 21.1%; SC, 10.5%). Median durations of CR/CRh or CR in the gilteritinib arm were not reached; the median duration of CRc in the gilteritinib arm was 9.2 months. Median durations of CR/CRh, CR, and CRc were not evaluable in the SC arm. In the overall ITT population, median durations of CR, CR/CRh, and CRc in the gilteritinib arm were 14.8, 11.0, and 4.6 months, respectively; median durations of CR and CR/CRh in the SC arm were both 1.8 months; the median duration of CRc was not evaluable.
Transplantation rates
In the overall ITT population, more patients assigned to gilteritinib (n = 63/247; 25.5%) than those assigned to SC (n = 19/124; 15.3%) underwent HSCT during the study. In the Japanese subgroup, 12 patients (36.4%) assigned to gilteritinib and 2 (13.3%) assigned to SC underwent HSCT. Nine Japanese patients who underwent HSCT after initial gilteritinib treatment resumed gilteritinib after transplantation.
Safety and tolerability
Most patients in the Japanese SAF (N = 47) experienced TEAEs (Table 4; gilteritinib, 100% [n = 33/33]; SC, 93% [n = 13/14]). Drug-related TEAEs occurred in 88% of patients treated with gilteritinib and in 71% of patients treated with SC. Drug-related TEAEs leading to gilteritinib discontinuation occurred in three patients (hyperglycemia, intestinal ischemia, sepsis, back pain, depressed consciousness [n = 1]; cholecystitis [n = 1]; pneumonia [n = 1]). Drug-related TEAEs leading to discontinuation of SC occurred in one patient (febrile neutropenia, diabetes mellitus, and delirium). Serious drug-related TEAEs occurring in ≥ 2 gilteritinib-treated patients were febrile neutropenia (n = 3), increased alanine aminotransferase (ALT; n = 3), increased aspartate aminotransferase (AST; n = 3), and pneumonia (n = 2). Five gilteritinib-treated patients died from TEAEs: AML progression (n = 2); intestinal ischemia, sepsis, depressed consciousness (n = 1); pancreatitis (n = 1); and pneumonia (n = 1). Two deaths stemmed from drug-related TEAEs (intestinal ischemia, sepsis, depressed consciousness [n = 1]; pneumonia [n = 1]).
Gilteritinib was generally well tolerated in this Japanese subgroup. Although gilteritinib exposure was approximately five times longer than SC exposure, the exposure-adjusted incidence of TEAEs was lower with gilteritinib (53.84/PY) than with SC (93.08/PY; Table 4), including all drug-related TEAEs leading to discontinuation (gilteritinib, 0.38/PY; SC, 2.31/PY) and grade ≥ 3 drug-related TEAEs (gilteritinib, 6.27/PY; SC, 26.92/PY). In the Japanese subgroup, incidences of TEAEs (100%) and deaths (55%) in gilteritinib-treated patients were similar to those observed in the overall SAF population (100% and 69%, respectively).
The most common TEAEs in gilteritinib-treated patients were febrile neutropenia (55%), increased AST (52%), increased ALT (46%), anemia (39%), constipation (39%), increased blood creatinine phosphokinase (39%), decreased platelet count (39%), and nausea (36%) (Table 5). Most common TEAEs in patients treated with SC were anemia (57%), decreased platelet count (50%), and decreased white blood cell count (36%). Higher incidences of grade ≥ 3 febrile neutropenia (55%) and neutropenia (12%) were seen with gilteritinib versus SC (29% and 7%, respectively). Lower incidences of grade ≥ 3 anemia (33%) and thrombocytopenia (9%) were observed with gilteritinib versus SC (57% and 21%, respectively).
Most common grade ≥ 3 drug-related TEAEs observed with gilteritinib treatment in the Japanese subgroup were febrile neutropenia (36%), decreased platelet count (27%), and anemia (24%) (Table 6). When comparing the Japanese subgroup to all other ADMIRAL patients, no clinically significant TEAEs were unique to Japanese patients.
Overall, seven gilteritinib-treated patients and two SC-treated patients in the Japanese subgroup had a maximum postbaseline mean Fridericia-corrected QT interval (QTcF) increase of > 30 ms; two patients treated with gilteritinib had a maximum postbaseline mean QTcF increase of > 60 ms. One case of prolonged QTcF occurred with gilteritinib. As no maximum postbaseline QTcF increases of > 500 ms occurred, no gilteritinib dose reductions or interruptions were required. No cases of Torsade de Pointes were observed in the entire SAF population.
Transfusion status and hospitalization in patients treated with gilteritinib
Among gilteritinib-treated Japanese patients, 22 were transfusion-dependent (TD) and 11 were transfusion-independent (TI) at baseline. Six of 22 TD patients (27%) at baseline became TI during the postbaseline period. Eight of 11 (73%) TI patients at baseline remained TI postbaseline.
Hospitalization rates during gilteritinib therapy were 94% in the Japanese subgroup and 85% in the overall ITT population; most hospitalizations (~ 96%) in both populations were outside the intensive care unit. While Japanese patients had longer hospitalization duration (median, 133 days; range 10–497 days) than the overall ITT population (median, 28 days; range 1–214 days), hospitalizations due to TEAEs were less frequent (55% vs 76%, respectively).
Discussion
The utility of targeting FLT3 mutations in AML has gained momentum based on findings from clinical trials [22, 25, 26, 28, 34] and the approval of FLT3-targeted agents [27, 35]. Results from the QuANTUM-R and ADMIRAL studies provide compelling evidence that targeting FLT3 improves response rate compared with SC in patients with R/R FLT3mut+ AML [22, 28]. However, it is important to note that the ADMIRAL trial enrolled patients with either FLT3-ITD and/or FLT3-TKD mutations due to gilteritinib’s activity against both mutation types, and included patients who had relapsed within or after 6 months following initial therapy [28]. The QuANTUM-R trial only enrolled patients with FLT3-ITD mutations who had relapsed within 6 months of initial therapy [22]. While findings from single-arm studies of gilteritinib and quizartinib in Japanese R/R AML patients have been reported [29, 36], this is the first comparative evaluation of gilteritinib versus conventional SC in a Japanese R/R AML cohort.
In the overall study population, patients randomized to gilteritinib had significantly longer OS and numerically higher rates of CR/CRh compared with patients randomized to SC [28]. The Japanese subgroup exhibited a similar trend, with numerically longer OS and improved CR/CRh rates compared with SC (49% vs 13%, respectively). In contrast to the overall study population (40%), most Japanese patients (60%) had been preselected for low-intensity chemotherapy. Overall, 79% of Japanese patients in the SC arm received low-intensity chemotherapy and none of these patients achieved CR/CRh. Findings related to safety and tolerability in the Japanese subgroup were generally consistent with those of the overall study population [28].
Exposure to gilteritinib was longer in Japanese patients (median, 5.1 months) than in the overall study population (median, 4.1 months) and a greater proportion of Japanese patients required dose reductions (39%) or interruptions (67%) compared with the overall study population (31% and 50%, respectively). The median duration of hospitalization was also longer in the Japanese subgroup (133 days) compared with the overall study population (28 days), which may partially stem from the fact that extended hospitalization does not necessarily increase medical expenses for Japanese patients eligible for the high-cost medical expense benefit issued by the Japan MHLW. Notably, oral gilteritinib therapy allows for outpatient treatment, which could potentially lower treatment costs and improve quality of life.
A phase 2 study of quizartinib in Japanese patients with R/R FLT3-ITD + AML reported a similar rate of CRc (54%) to that observed with gilteritinib (58%) in the Japanese subgroup from the ADMIRAL trial [36]. However, CRi was the most frequent response (48%) with quizartinib in the Japanese phase 2 study, with no patients achieving CR [36]. In contrast, 24% of Japanese patients achieved CR with gilteritinib in the ADMIRAL trial. A higher incidence of QT prolongation (35%) was observed with quizartinib [36] compared with gilteritinib (< 10%), whereas liver enzyme (AST/ALT) elevations occurred more frequently with gilteritinib (46–52%) than with quizartinib (< 10–11%). Although Japanese patients treated with gilteritinib in the ADMIRAL trial had a higher incidence of grade ≥ 3 febrile neutropenia (55%) than those who received quizartinib in the Japanese phase 2 study (43%), rates of grade ≥ 3 anemia (33% vs 27%) and thrombocytopenia (9% vs 11%) were similar [36].
As is the nature of subgroup analyses, this analysis was restricted to a small, rather homogenous patient subset, without adjustment for multiple comparisons. Baseline characteristics were less balanced in the Japanese subgroup compared with the overall study population. As most Japanese patients treated with SC were aged > 65 years (87%) and received low-intensity chemotherapy (79%), a potential bias toward poorer outcomes with SC treatment in this Japanese subgroup cannot be ruled out. Thus, results from this analysis should be interpreted with caution.
Single-agent gilteritinib therapy improved response rates in Japanese patients with R/R FLT3mut+ AML and resulted in numerically longer survival compared with SC. The observed trends in treatment efficacy and safety are consistent with the overall ADMIRAL study population and confirm that gilteritinib is an important treatment option for the Japanese R/R FLT3mut+ AML population. Investigations of gilteritinib as part of induction/consolidation (NCT02236013 and NCT04027309) and as post-consolidation/post-HSCT maintenance therapy (NCT02927262 and NCT02997202) are ongoing.
Data availability
Researchers may request access to anonymized participant level data, trial level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com.
For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
References
Niino M, Matsuda T (2016) Type distribution of myeloid leukemia from Cancer Incidence in Five Continents Vol. X. Jpn J Clin Oncol 46(4):394
Miyawaki S (2012) Clinical studies of acute myeloid leukemia in the Japan Adult Leukemia Study Group. Int J Hematol 96(2):171–177
Breems DA, Van Putten WL, Huijgens PC et al (2005) Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol 23(9):1969–1978
Ganzel C, Sun Z, Cripe LD et al (2018) Very poor long-term survival in past and more recent studies for relapsed AML patients: The ECOG-ACRIN experience. Am J Hematol 93:1074–1081
Kihara R, Nagata Y, Kiyoi H et al (2014) Comprehensive analysis of genetic alterations and their prognostic impacts in adult acute myeloid leukemia patients. Leukemia 28(8):1586–1595
Levis M (2017) Midostaurin approved for FLT3-mutated AML. Blood 129(26):3403–3406
Kiyoi H, Towatari M, Yokota S et al (1998) Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia 12(9):1333–1337
Sakaguchi M, Yamaguchi H, Kuboyama M et al (2019) Significance of FLT3-tyrosine kinase domain mutation as a prognostic factor for acute myeloid leukemia. Int J Hematol 110(5):566–574
Yamamoto Y, Kiyoi H, Nakano Y et al (2001) Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood 97(8):2434–2439
Bacher U, Haferlach C, Kern W et al (2008) Prognostic relevance of FLT3-TKD mutations in AML: the combination matters—an analysis of 3082 patients. Blood 111(5):2527
Yokota S, Kiyoi H, Nakao M et al (1997) Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia 11(10):1605–1609
Chevallier P, Labopin M, Turlure P et al (2011) A new leukemia prognostic scoring system for refractory/relapsed adult acute myelogeneous leukaemia patients: a GOELAMS study. Leukemia 25(6):939–944
Papaemmanuil E, Gerstung M, Bullinger L et al (2016) Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 374(23):2209–2221
Wattad M, Weber D, Dohner K et al (2017) Impact of salvage regimens on response and overall survival in acute myeloid leukemia with induction failure. Leukemia 31(6):1306–1313
Smith CC, Lin K, Stecula A et al (2015) FLT3 D835 mutations confer differential resistance to type II FLT3 inhibitors. Leukemia 29(12):2390–2392
Smith CC, Wang Q, Chin CS et al (2012) Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature 485(7397):260–263
Daver N, Schlenk RF, Russell NH et al (2019) Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia 33(2):299–312
Smith BD, Levis M, Beran M et al (2004) Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood 103(10):3669–3676
Fiedler W, Serve H, Dohner H et al (2005) A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood 105(3):986–993
Fischer T, Stone RM, Deangelo DJ et al (2010) Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol 28(28):4339–4345
O’Farrell AM, Yuen HA, Smolich B et al (2004) Effects of SU5416, a small molecule tyrosine kinase receptor inhibitor, on FLT3 expression and phosphorylation in patients with refractory acute myeloid leukemia. Leuk Res 28(7):679–689
Cortes JE, Khaled S, Martinelli G et al (2019) Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 20:984
Galanis A, Levis M (2015) Inhibition of c-Kit by tyrosine kinase inhibitors. Haematologica 100(3):e77-79
Lee LY, Hernandez D, Rajkhowa T et al (2017) Preclinical studies of gilteritinib, a next-generation FLT3 inhibitor. Blood 129(2):257–260
Mori M, Kaneko N, Ueno Y et al (2017) Gilteritinib, a FLT3/AXL inhibitor, shows antileukemic activity in mouse models of FLT3 mutated acute myeloid leukemia. Invest New Drugs 35(5):556–565
Perl AE, Altman JK, Cortes J et al (2017) Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol 18(8):1061–1075
Pharmaceutical Evaluation Division, Pharmaceutical Safety and Environmental Health Bureau. Ministry of Health, Labour and Welfare. Report on the Deliberation Results. https://www.pmda.go.jp/files/000228502.pdf. Accessed July 20, 2020. (2018)
Perl AE, Martinelli G, Cortes JE et al (2019) Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med 381(18):1728–1740
Usuki K, Sakura T, Kobayashi Y et al (2018) Clinical profile of gilteritinib in Japanese patients with relapsed/refractory acute myeloid leukemia: an open-label phase 1 study. Cancer Sci 109(10):3235–3244
Murphy KM, Levis M, Hafez MJ et al (2003) Detection of FLT3 internal tandem duplication and D835 mutations by a multiplex polymerase chain reaction and capillary electrophoresis assay. J Mol Diagn 5(2):96–102
Levis M, Ravandi F, Wang ES et al (2011) Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood 117(12):3294–3301
Jackson G, Taylor P, Smith GM et al (2001) A multicentre, open, non-comparative phase II study of a combination of fludarabine phosphate, cytarabine and granulocyte colony-stimulating factor in relapsed and refractory acute myeloid leukaemia and de novo refractory anaemia with excess of blasts in transformation. Br J Haematol 112(1):127–137
Cheson BD, Bennett JM, Kopecky KJ et al (2003) Revised recommendations of the International working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 21(24):4642–4649
Stone RM, Mandrekar SJ, Sanford BL et al (2017) Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 377(5):454–464
PMDA Quizartinib Approval. https://www.pmda.go.jp/files/000235289.pdf. Accessed March 3, 2020
Takahashi T, Usuki K, Matsue K et al (2019) Efficacy and safety of quizartinib in Japanese patients with FLT3-ITD positive relapsed or refractory acute myeloid leukemia in an open-label, phase 2 study. Int J Hematol 110(6):665–674
Acknowledgements
We would like to acknowledge all patients and their families for their participation in this study. In addition, we also acknowledge the investigators, coordinators, and site personnel involved in this study. This study was funded by Astellas Pharma, Inc. Medical writing/editorial assistance was provided by Patrick Tucker, PhD, Kalpana Vijayan, PhD, and Elizabeth Hermans, PhD, from Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and funded by the study sponsor.
Author information
Authors and Affiliations
Contributions
NH from AP US, Inc. and MK from AP, Inc. contributed to the study design along with SM and TN. Data analyses were performed by QL, MK, EB, and NH. All authors were involved in data acquisition and interpretation, and in the development and critical review of the manuscript draft.
Corresponding author
Ethics declarations
Conflict of interest
SF has received personal fees from Astellas Pharma, Celgene, Chugai Pharmaceutical, Eisai, Kyowa Hakko Kirin, Nippon Shinyaku, Novartis, Ortho Clinical Diagnostics, Otsuka, and Pfizer, all outside the submitted work. SC has received financial support from Astellas Pharma, Ono Pharmaceutical Co, Kyowa-Kirin, Takeda Pharmaceuticals, Sanofi, Bristol-Myers Squibb, and Chugai Pharmaceutical. M Kizaki has received personal fees from Bristol-Myers Squibb, Celgene, Novartis Pharma, Janssen Pharma, and Sumitomo Dainippon Pharma, all outside the submitted work. M Kizaki also received grants and personal fees from Takeda, Kyowa Kirin, and Ono Pharmaceutical, as well as grants from Chugai Pharmaceutical and Daiichi-Sankyo, all outside the submitted work. TN has received personal fees from Eisai, Astellas Pharma, Nippon Shinyaku, Bristol-Myers Squibb, and Sysmex, all outside the submitted work. Y Maeda has received honoraria from Mundipharma, Kyowa-Kirin, Bristol-Myers Squibb, Chugai Pharma, Pfizer, Celgene, Novartis, and Pfizer. Y Maeda also received research funding from Astellas Pharma, Bristol-Myers Squibb, Takeda, Kyowa Kirin, and Chugai Pharmaceutical. TS has received grants from Astellas Pharma during the conduct of the study. TS also received personal fees from Eisai, Novartis, Kyowa Kirin, Takeda Pharmaceutical, Celgene, Bristol-Myers Squibb, Ono Pharmaceutical, Pfizer, Janssen Pharmaceutical, and Otsuka Pharmaceutical, all outside the submitted work. KU has received grants and personal fees from Astellas Pharma, Alexion Pharmaceuticals, AbbVie, Gilead, SymBio Pharmaceuticals, Daiichi Sankyo, Otsuka Pharmaceutical, Novartis, Bristol-Myers Squibb, Ono Pharmaceutical, Celgene, Takeda Pharmaceutical, Nippon, Boehringer Ingelheim, and Kyowa Kirin. KU also received grants from Sumitomo Dainippon Pharma, Chugai Pharmaceutical, Janssen Pharmaceutical, Mundipharma, Astellas-Amgen-Biopharma, Apellis Pharmaceuticals, Nippon Shinyaku, and Pfizer. KU also received personal fees from Eisai, MSD, SymBio Pharmaceuticals, PharmaEssentia Corp., and Yakult Honsha, all outside the submitted work. YK received grants, personal fees, and other support from Pfizer; YK also received personal fees from Astellas and Symbio, all outside the submitted work. JK has received other support from Astellas during the conduct of the study; JK has also received grants and personal fees from Astellas outside the submitted work. M Kusano, QL, EB, and N Hasabou are all employees of Astellas Pharma. N Hosono, HY, NA, KA, KK, TH, MH, MN, TI, MO, and Y Morita have no financial relationships to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hosono, N., Yokoyama, H., Aotsuka, N. et al. Gilteritinib versus chemotherapy in Japanese patients with FLT3-mutated relapsed/refractory acute myeloid leukemia. Int J Clin Oncol 26, 2131–2141 (2021). https://doi.org/10.1007/s10147-021-02006-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-02006-7