Abstract
Background
The clinical and pathological features of sporadic microsatellite instability-high (MSI) colorectal cancer (CRC) are still unclear. The present study aimed to clarify the clinicopathological features of sporadic MSI CRC in comparison with those of Lynch syndrome (LS) exploratorily.
Methods
The present study was a single-center, retrospective cohort study. Sporadic MSI CRC was defined as MSI CRC with aberrant promoter hypermethylation of the MLH1 gene, while hereditary MSI CRC was defined colorectal cancer in patients with LS.
Results
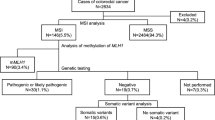
In total, 2653 patients were enrolled; of these, 120 (4.5%) had MSI CRC, 98 had sporadic MSI CRC, and 22 had LS. Patients with sporadic MSI CRC were significantly older (p < 0.001) than those with LS and had a right-sided colonic tumor (p < 0.001) which was pathologically poorly differentiated or mucinous (p = 0.025). The overall survival rate was significantly lower in patients with stage I, II or III MSI CRC than in those with LS (p = 0.024). However, the recurrence-free survival rate did not differ significantly (p = 0.85).
Conclusions
We concluded that patients with sporadic MSI are significantly older, tumors more likely to locate in the right-sided colon, pathologically poorly differentiated or mucinous, and worse overall survival than in those with LS.



Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30. https://doi.org/10.3322/caac.21442
Dienstmann R, Salazar R, Tabernero J (2014) The Evolution of Our Molecular Understanding of Colorectal Cancer: What We Are Doing Now, What the Future Holds, and How Tumor Profiling Is Just the Beginning. Am Soc Clin Oncol Educ Book 34:91–99. https://doi.org/10.14694/edbook_am.2014.34.91
Devaud N, Gallinger S (2013) Chemotherapy of MMR-deficient colorectal cancer. FamCancer 12:301–306. https://doi.org/10.1007/s10689-013-9633-z
Geiersbach KB, Samowitz WS (2011) Microsatellite instability and colorectal cancer. Arch Pathol Lab Med 135:1269–1277. https://doi.org/10.5858/arpa.2011-0035-RA
Canard G, Lefevre JH, Colas C et al (2012) Screening for lynch syndrome in colorectal cancer: are we doing enough? Ann Surg Oncol 19:809–816. https://doi.org/10.1245/s10434-011-2014-7
Ribic CM, Sargent DJ, Moore MJ et al (2003) Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 349:247–257. https://doi.org/10.1056/nejmoa022289
Ward R (2001) Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut 48:821–829. https://doi.org/10.1136/gut.48.6.821
Shitoh K, Konishi F, Miyakura Y et al (2002) Microsatellite instability as a marker in predicting metachronous multiple colorectal carcinomas after surgery: a cohort-like study. Dis Colon Rectum 45:329–333. https://doi.org/10.1007/s10350-004-6177-1
Natsume S, Yamaguchi T, Takao M et al (2018) Clinicopathological and molecular differences between right-sided and left-sided colorectal cancer in Japanese patients. Jpn J Clin Oncol 48:609–618. https://doi.org/10.1093/jjco/hyy069
Perucho M, Boland CR, Thibodeau SN et al (1998) Correspondence re: C. R. Boland et al., A national cancer institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in color. Cancer Res 59:5248–5257
Ogino S, Kawasaki T, Brahmandam M et al (2006) Precision and performance characteristics of bisulfite conversion and real-time PCR ( MethyLight ) for quantitative DNA methylation analysis. J Mol Diagnostics 8:209–217. https://doi.org/10.2353/jmoldx.2006.050135
Asaka SI, Arai Y, Nishimura Y et al (2009) Microsatellite instability-low colorectal cancer acquires a KRAS mutation during the progression from Dukes’ A to Dukes’ B. Carcinogenesis 30:494–499. https://doi.org/10.1093/carcin/bgp017
Nosho K, Kure S, Irahara N et al (2009) A prospective cohort study shows unique epigenetic, genetic, and prognostic features of synchronous colorectal cancers. Gastroenterology 137:1–23. https://doi.org/10.1053/j.gastro.2009.08.002
Vilar E, Gruber SB (2010) Microsatellite instability in colorectal cancer—the stable evidence. Nat Rev Clin Oncol 7:153–162
Matloff J, Lucas A, Polydorides AD, Itzkowitz SH (2013) Molecular tumor testing for lynch syndrome in patients with colorectal cancer. JNCCN J Natl Compr Cancer Netw 11:1380–1385. https://doi.org/10.6004/jnccn.2013.0161
Das C, Lucia MSHK, TJ, (2017) Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev 26:404–412
Li D, Hoodfar E, Jiang SF et al (2019) Comparison of universal versus age-restricted screening of colorectal tumors for lynch syndrome using mismatch repair immunohistochemistry: a cohort study. Ann Intern Med 171:19–26
Adar T, Rodgers LH, Shannon KM et al (2018) Universal screening of both endometrial and colon cancers increases the detection of Lynch syndrome. Cancer 124:3145–3153. https://doi.org/10.1002/cncr.31534
Chika N, Eguchi H, Kumamoto K et al (2017) Prevalence of Lynch syndrome and Lynch-like syndrome among patients with colorectal cancer in a Japanese hospital-based population. Jpn J Clin Oncol 47:108–117. https://doi.org/10.1093/jjco/hyw178
Jiang W, Cai MY, Li SY et al (2019) Universal screening for Lynch syndrome in a large consecutive cohort of Chinese colorectal cancer patients: High prevalence and unique molecular features. Int J Cancer 144:2161–2168. https://doi.org/10.1002/ijc.32044
Moreira L, Balaguer F, Lindor N et al (2012) Identification of Lynch syndrome among patients with colorectal cancer. JAMA-J Am Med Assoc 308:1555–1565. https://doi.org/10.1001/jama.2012.13088
Shia J (2008) Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: Part I. The utility of immunohistochemistry. J Mol Diagnostics 10:293–300. https://doi.org/10.2353/jmoldx.2008.080031
Yamada R, Yamaguchi T, Iijima T et al (2018) Differences in histological features and PD-L1 expression between sporadic microsatellite instability and Lynch-syndrome-associated disease in Japanese patients with colorectal cancer. Int J Clin Oncol 23:504–513
Slattery ML, Curtin PDK, Wolff PDRK et al (2009) A comparison of colon and rectal somatic DNA 0terations. Dis colon rectum. 52:1304–1311. https://doi.org/10.1007/DCR.0b013e3181a0e5df
Barault L, Funes M, Vega D et al (2008) Hypermethylator Phenotype in Sporadic Colon Cancer : Study on a Population-Based Series of 582 Cases. Cancer res 68:8541–8547. https://doi.org/10.1158/0008-5472.CAN-08-1171
Nosho K, Irahara N, Shima K et al (2008) Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS ONE 3:e3698. https://doi.org/10.1371/journal.pone.0003698
Liu GC, Liu RY, Yan JP et al (2018) The heterogeneity between lynch-associated and sporadic mmr deficiency in colorectal cancers. J Natl Cancer Inst 110:975–984. https://doi.org/10.1093/jnci/djy004
Liu Y, Sethi NS, Hinoue T et al (2018) Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell 33:721–735. https://doi.org/10.1016/j.ccell.2018.03.010
Niwa T, Tsukamoto T, Toyoda T et al (2010) Inflammatory processes triggered by Helicobacter pylori infection cause aberrant DNA methylation in gastric epithelial cells. Cancer Res 70:1430–1440. https://doi.org/10.1158/0008-5472.CAN-09-2755
Niwa T, Ushijima T (2010) Induction of epigenetic alterations by chronic inflammation and its significance on carcinogenesis. Elsevier Inc., Lyon
Nosho K, Sukawa Y, Adachi Y et al (2016) Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol 22:557–566. https://doi.org/10.3748/wjg.v22.i2.557
Mima K, Nishihara R, Rong Qian Z et al (2016) Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis were responsible for collection of tumour tissue, and acquisition of epidemiologic, clinical and tumour tissue data, including histopathological and immunohistochemical character. Gut 65:1973–1980
Tsai YJ, Huang SC, Lin HH et al (2018) Differences in gene mutations according to gender among patients with colorectal cancer. World J Surg Oncol 16:1–5. https://doi.org/10.1186/s12957-018-1431-5
Slattery ML, Potter JD, Curtin K et al (2001) Estrogens reduce and withdrawal of estrogens increase risk of microsatellite instability-positive colon cancer. Cancer Res 61:126–130
Maccaroni E, Bracci R, Giampieri R, et al (2015) Prognostic impact of mismatch repair genes germline defects in colorectal cancer patients: Are all mutations equal? Oncotarget 6:38737–38748. https://doi.org/10.18632/oncotarget.5395
Sinicrope FA, Foster NR, Thibodeau SN et al (2011) DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst 103:863–875. https://doi.org/10.1093/jnci/djr153
Jover R, Nguyen TP, Pérez-Carbonell LZ et al (2011) 5-fluorouracil adjuvant chemotherapy does not increase survival in patients with CpG island methylator phenotype colorectal cancer. Gastroenterology 140:1174–1181
Dkk T, Burgermeister E, Ph D et al (2012) TFAP2E–DKK4 and chemoresistance in colorectal cancer. N Engl J Med 366:44–53
Perez-Carbonell L, Balaguer F, Toiyama Y et al (2014) IGFBP3 methylation is a novel diagnostic and predictive biomarker in colorectal cancer. PLoS ONE 9:1–11. https://doi.org/10.1371/journal.pone.0104285
André T, Shiu K-K, Kim TW et al (2020) Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N Engl J Med 383:2207–2218. https://doi.org/10.1056/nejmoa2017699
Marabelle A, Le DT, Ascierto PA et al (2020) Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/ mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 38:1–10. https://doi.org/10.1200/JCO.19.02105
Overman MJ, Lonardi S, Wong KYM et al (2018) Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol 36:773–779. https://doi.org/10.1200/JCO.2017.76.9901
Kang SY, Park CK, Chang DK et al (2015) Lynch-like syndrome: characterization and comparison with EPCAM deletion carriers. Int J Cancer 136:1568–1578. https://doi.org/10.1002/ijc.29133
Mensenkamp AR, Vogelaar IP, Van Zelst-Stams WAG et al (2014) Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in lynch syndrome-like tumors. Gastroenterology 146:643-646.e8. https://doi.org/10.1053/j.gastro.2013.12.002
Acknowledgements
We wish to express our gratitude to the Office of Metropolitan Hospital Management, Tokyo Metropolitan Government for their support. We are grateful to all the patients and their families for their participation in our study and would like to thank Mr. James R. Valera for his assistance in editing this manuscript.
Funding
The Office of Metropolitan Hospital Management, Tokyo Metropolitan Government provided funding for this study.
Author information
Authors and Affiliations
Contributions
YN, TI, TI, EK, MT, AT, SH, and TH: substantial contributions to the conception and design of the study and acquisition, analysis or interpretation of data. YN and TY: drafting or critical revision of the manuscript for important intellectual content. TY: final approval of the version to be published.
Corresponding author
Ethics declarations
Conflicts of interest
All the authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nakayama, Y., Iijima, T., Inokuchi, T. et al. Clinicopathological features of sporadic MSI colorectal cancer and Lynch syndrome: a single-center retrospective cohort study. Int J Clin Oncol 26, 1881–1889 (2021). https://doi.org/10.1007/s10147-021-01968-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-01968-y