Abstract
Background
Recent studies have shown that immune-related adverse events (irAEs) caused by immune checkpoint inhibitors (ICIs) were correlated with favorable clinical outcome in patients with melanoma. However, in metastatic renal cell carcinoma (mRCC) patients, there have been few reports about the correlation between irAEs and clinical efficacy of anti-programmed cell death protein-1 (PD-1) therapy.
Methods
We retrospectively investigated 160 mRCC patients who started nivolumab monotherapy between September 2016 and July 2019. IrAEs were defined as patients’ AEs having a potential immunological basis that required close follow-up, or immunosuppressive therapy. We compared the data of patients who received nivolumab into two groups based on the occurrence of irAEs and assessed clinical efficacy in both groups.
Results
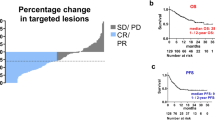
Of all mRCC patients, 47 patients (29.4%) developed irAEs. In patients who developed irAEs, the objective response rate and disease control rate were 38.8% and 77.6%, which were significantly higher when compared to that in patients without irAEs (p = 0.012 and p < 0.001, respectively). Furthermore, the incidence of irAEs was significantly associated with an increase in progression-free survival (PFS) [Hazard ratio (HR) = 0.4867; p = 0.0006] and overall survival (OS) (HR = 0.526; p = 0.0252). Importantly, PFS and OS seemed to be similar in patients who discontinued treatment because of irAEs and in those who did not discontinue because of irAEs (p = 0.36 and p = 0.35, respectively).
Conclusion
Development of irAEs strongly correlates with clinical benefit for mRCC patients receiving nivolumab monotherapy in real-world settings.




Similar content being viewed by others
Abbreviations
- ccRCC:
-
Clear cell renal carcinoma
- CTCAE:
-
Common Terminology Criteria for Adverse Event
- DCR:
-
Disease control rate
- ICI:
-
Immune checkpoint inhibitor
- iRAEs:
-
Immune-related adverse events
- mRCC:
-
Metastatic renal cell carcinoma
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PD-1:
-
Programmed death 1
- PD-L1:
-
Programmed death-ligand 1
- PFS:
-
Progression-free survival
- RCC:
-
Renal cell carcinoma
References
Bray FFJ, Soerjomataram I, Siegel RL et al (2020) Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 70:313
Rizvi NA, Hellmann MD, Snyder A et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124–128
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Postow MA, Callahan MK, Wolchok JD (2015) Immune checkpoint blockade in cancer therapy. J Clin Oncol 33:1974–1982
Motzer RJ, Escudier B, McDermott DF et al (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803–1813
Tomita Y, Fukasawa S, Shinohara N et al (2017) Nivolumab versus everolimus in advanced renal cell carcinoma: Japanese subgroup analysis from the CheckMate 025 study. Jpn J Clin Oncol 47:639–646
Verzoni E, Carteni G, Cortesi E et al (2019) Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: the Italian expanded access program. J Immunother Cancer 7:99
Yip SM, Wells C, Moreira R et al (2018) Checkpoint inhibitors in patients with metastatic renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Cancer 124:3677–3683
Hinata N, Yonese J, Masui S et al (2020) A multicenter retrospective study of nivolumab monotherapy in previously treated metastatic renal cell carcinoma patients: interim analysis of Japanese real-world data. Int J Clin Oncol. https://doi.org/10.1007/s10147-020-01692-z
Masuda K, Shoji H, Nagashima K et al (2019) Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer 19:974
Nakamura Y, Tanaka R, Asami Y et al (2017) Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol 44:117–122
Okada N, Kawazoe H, Takechi K et al (2019) Association between immune-related adverse events and clinical efficacy in patients with melanoma treated with nivolumab: a multicenter retrospective study. Clin Ther 41:59–67
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359:1350–1355
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378:158–168
Sato K, Akamatsu H, Murakami E et al (2018) Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 115:71–74
Freeman-Keller M, Kim Y, Cronin H et al (2016) Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 22:886–894
Haratani K, Hayashi H, Chiba Y et al (2018) Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4:374–378
Schadendorf D, Wolchok JD, Hodi FS et al (2017) Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol 35:3807–3814
Verzoni E, Cartenì G, Cortesi E et al (2019) Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: the Italian expanded access program. J Immunother Cancer 7:99
Ishihara H, Takagi T, Kondo T et al (2019) Association between immune-related adverse events and prognosis in patients with metastatic renal cell carcinoma treated with nivolumab. Urol Oncol 37:355.e321-e329
Kato T, Tomiyama E, Koh Y et al (2020) A potential mechanism of anticancer immune response coincident with immune-related adverse events in patients with renal cell carcinoma. Anticancer Res 40:4875–4883
Hodi FS, Chiarion-Sileni V, Gonzalez R et al (2018) Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 19:1480–1492
Pollack MH, Betof A, Dearden H et al (2018) Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol 29:250–255
Simonaggio A, Michot JM, Voisin AL et al (2019) Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.1022
Weber JS, Hodi FS, Wolchok JD et al (2017) Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 35:785–792
Author information
Authors and Affiliations
Contributions
TK was responsible for the study conception and design, and conducted the study, data collection and analysis, drafting the manuscript. AN, NK, WN, TS, KM, KH, AK, TU, RI, KN, SY, TT, MT, ST, KN, NN, and MU conducted the study, data collection and analysis, drafting the manuscript, and manuscript revision. All authors read and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kato, T., Nagahara, A., Kawamura, N. et al. The prognostic impact of immune-related adverse events in metastatic renal cell carcinoma patients treated with nivolumab: a real-world multi-institutional retrospective study. Int J Clin Oncol 26, 954–961 (2021). https://doi.org/10.1007/s10147-021-01872-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-01872-5