Abstract
Background
The standard regimen of systemic chemotherapy for patients with advanced urothelial cancer (UC) changed from methotrexate, vinblastine, adriamycin, and cisplatin (MVAC) to gemcitabine and cisplatin (GC) in 2008 when the use of gemcitabine for UC began to be reimbursed by public health insurance in Japan. We examined its influence on the chemotherapy trend in elderly patients aged ≥80 years.
Methods
Among 345 patients included in our previous multicenter retrospective cohort study (chemotherapy for urothelial carcinoma: renal function and efficacy study; CURE study), the outcome of 30 patients aged ≥80 years was reviewed before and after 2008 and compared with 315 young patients.
Results
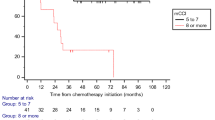
There were only 7 (4.6 %) elderly individuals among all registered patients before 2008, whereas the number increased to 23 (12 %) after 2008. Before 2008, only one elderly patient received MVAC, while GC (whose rate was similar to the rate in young patients) was administered to 13 patients (56.5 %) after 2008. The chemotherapeutic effect and overall survival (OS) rate was not significantly different between young and elderly patients. In the elderly treated with the GC regimen, the renal impairment rate after the first cycle was significantly higher, and the presence of distant metastases and renal impairment were independent prognostic factors in a multivariate analysis.
Conclusion
Since GC was approved as the standard regimen for first-line chemotherapy in UC, selected elderly patients have been able to safely receive systemic chemotherapy like young patients. The clinical response rate and OS rate were similar to the young, but we need to monitor changes in renal function more closely in the elderly treated with GC.

Similar content being viewed by others
Abbreviations
- UC:
-
Urothelial carcinoma
- GC:
-
Gemcitabine and cisplatin
- MVAC/MEC:
-
Methotrexate, vinblastine, adriamycin, and cisplatin/methotrexate, epirubicin, and cisplatin
- GP:
-
Gemcitabine and paclitaxel
- CR:
-
Complete response
- PR:
-
Partial response
- PD:
-
Progressive disease
- NC:
-
No change
- MDRD:
-
Modification of Diet in Renal Disease
- PS:
-
Performance status
References
Serrano M, Blasco MA (2007) Cancer and ageing: convergent and divergent mechanisms. Nat Rev Mol Cell Biol 8:715–722
Surveillance Research Program SEER stat fact sheets: Bladder Cancer. National Cancer Institute. http://seer.cancer.gov/statfacts/html/urinb.html
Rose TL, Milowsky MI (2015) Management of muscle-invasive bladder cancer in the elderly. Curr Opin Urol 25:459–467
Sternberg CN, Yagoda A, Scher HI et al (1988) M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for advanced transitional cell carcinoma of the urothelium. J Urol 139:461–469
Duthie E (2004) Physiology of age: relevance to symptoms, perceptions, and treatment tolerance. In: Balducci L, Lyman GH, Ershler WB et al (eds) Comprehensive geriatric oncology. Taylor and Francis, London, pp 207–223
Balducci L (2008) Cancer chemotherapy in the older person. In: Balducci L, Ershler B, DeGaetano G (eds) Blood disorders in the elderly. University Press, Cambridge, pp 225–236
von der Maase H, Hansen SW, Roberts JT et al (2000) Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18:3068–3077
Ichioka D, Miyazaki J, Inoue T et al (2015) Impact of renal function of patients with advanced urothelial cancer on eligibility for first-line chemotherapy and treatment outcomes. Jpn J Clin Oncol 45:867–873
Kikuchi E, Miyazaki J, Yuge K et al (2016) Do metastatic upper tract urothelial carcinoma and bladder carcinoma have similar clinical responses to systemic chemotherapy? A Japanese multi-institutional experience. Jpn J Clin Oncol 46:163–169
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Matsuo S, Imai E, Horio M et al (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992
Goto T, Yoshimura K, Matsui Y et al (2011) Impact of different methods of estimating renal function on determining eligibility for cisplatin (CDDP)-based chemotherapy in patients with invasive urothelial carcinoma. Hinyokika Kiyo 57:671–676
Kaag MG, O’Malley RL, O’Malley P et al (2010) Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol 58:581–587
Lane BR, Smith AK, Larson BT et al (2010) Chronic kidney disease after nephroureterectomy for upper tract urothelial carcinoma and implications for the administration of perioperative chemotherapy. Cancer 116:2967–2973
Balducci L, Cohen HJ, Engstrom PF et al (2005) Senior adult oncology clinical practice guidelines in oncology. JNCCN 3:572–590
Thyss A, Saudes L, Otto J et al (1994) Renal tolerance of cisplatin in patients more than 80 years old. J Clin Oncol 12:2121–2125
De Santis M, Bellmunt J, Mead G et al (2009) Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer “unfit” for cisplatin-based chemotherapy: phase II—results of EORTC study 30986. J Clin Oncol 27:5634–5639
De Santis M, Bellmunt J, Mead G et al (2012) Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 30:191–199
Carreca I, Balducci L (2009) Cancer chemotherapy in the older cancer patient. Urol Oncol 27:633–642
Guancial EA, Roussel B, Bergsma DP et al (2015) Bladder cancer in the elderly patient: challenges and solutions. Clin Interv Aging 10:939–949
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Matsui, Y., Ogawa, O., Ishitsuka, R. et al. Current status of systemic chemotherapy for octogenarians with advanced urothelial cancer in Japan: a Japanese multi-institutional study (CURE study) . Int J Clin Oncol 21, 1142–1149 (2016). https://doi.org/10.1007/s10147-016-1007-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1007-8