Abstract
Background
The outcome of surveillance for Japanese patients with clinical stage I testicular germ cell cancer (GCC) was investigated in the multi-detector computed tomography (MDCT) era.
Methods
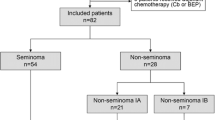
The medical records of 92 Japanese patients with stage I GCC, who received treatment in our institution between March 1999 and February 2013, were reviewed. As six patients requested and received prophylactic chemotherapy and two patients seriously deviated from surveillance schedule, these patients were excluded from the study. Data from a total 84 patients were analyzed,
Results
The median follow-up period following diagnosis was 5.1 years (inter-quartile range: IQR, 2.3–7.7 years). Of the 84 patients, eight (9.5 %) had a recurrence of their cancer in this observation period. Regarding histologic subtypes, the recurrence rates were five (9.3 %) of the 54 patients with seminoma and three (10 %) of the 30 patients with nonseminomatous germ cell tumor (NSGCT). All eight patients who experienced a recurrence did so within 2 years; they all underwent induction chemotherapy and remain alive at the time of writing, with no evidence of disease. Among 31 seminoma patients with a tumor more than 4 cm in size and rete testis invasion, cancer recurred in three (9.7 %) during the surveillance period. On the other hand, among the 13 patients with NSGCT and vascular invasion, three (23 %) experienced a recurrence, whereas the figure was zero for the 11 (0 %) patients without vascular invasion.
Conclusion
Fewer than 10 % of Japanese patients with stage I testicular GCC suffered a recurrence in the 5-year observation period of this study. The risk of occult disease, which will result in relapse, might be decreased in the MDCT era. All patients must be fully informed of the anticipated recurrence rate and the potential risks of exposure to chemotherapy agents.

Similar content being viewed by others
Abbreviations
- GCC:
-
Germ cell cancer
- NSGCT:
-
Nonseminomatous germ cell tumor
- MDCT:
-
Multi-detector computed tomography
- CT:
-
Computed tomography
- AFP:
-
Alpha-fetoprotein
- hCG:
-
Human chorionic gonadotropin
- LDH:
-
Lactate dehydrogenase
- BEP:
-
Bleomycin, etoposide, and cisplatin
References
Stephenson JA, Gilligan TD (2012) Neoplasms of the testis. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (eds) Campbell-Walsh urology, 10th edn. Saunders, New York, pp 837–870
Albers P, Albrecht W, Algaba F et al (2011) EAU guidelines on testicular cancer: 2011 update. Eur Urol 60:304–319
Klepp O, Flodgren P, Maartman-Moe H et al (1990) Early clinical stages (CS1, CS1Mk + and CS2A) of non-seminomatous testis cancer. Value of pre and postorchidectomy serum tumour marker information in prediction of retroperitoneal lymph node metastases. Swedish–Norwegian Testicular Cancer Project (SWENOTECA). Ann Oncol 1:281–288
Shinohara T, Ohyama S, Yamaguchi T et al (2005) Clinical value of multidetector row computed tomography in detecting lymph node metastasis of early gastric cancer. Eur J Surg Oncol 31:743–748
Kanamoto T, Matsuki M, Okuda J et al (2007) Preoperative evaluation of local invasion and metastatic lymph nodes of colorectal cancer and mesenteric vascular variations using multidetector-row computed tomography before laparoscopic surgery. J Comput Assist Tomogr 31:831–839
Nasu Y, Shikishima H, Miyasaka Y et al (2010) A study of the assessment of axillary lymph nodes before surgery for breast cancer using multidetector-row computed tomography. Surg Today 40:1023–1026
Cullen MH, Stenning SP, Parkinson MC et al (1996) Short-course adjuvant chemotherapy in high-risk stage I nonseminomatous germ cell tumors of the testis: a medical research council report. J Clin Oncol 14:1106–1113
Studer UE, Fey MF, Calderoni A et al (1993) Adjuvant chemotherapy after orchidectomy in high-risk patients with clinical stage I: non-seminomatous testicular cancer. Eur Urol 23:444–449
Maroto P, García del Muro X, Aparicio J et al (2005) Multicentre risk adapted management for stage I: non-seminomatous germ cell tumours. Ann Oncol 16:1915–1920
Tandstad T, Cohn-Cedermark G, Dahl O et al (2010) Long-term follow-up after risk-adapted treatment in clinical stage 1 (CS1) nonseminomatous germ-cell testicular cancer (NSGCT) implementing adjuvant CVB chemotherapy: a SWENOTECA study. Ann Oncol 21:1858–1863
Shelley MD, Burgon K, Mason MD (2002) Treatment of testicular germ-cell cancer: a Cochrane evidence-based systematic review. Cancer Treat Rev 28:237–253
Oliver RT, Mead GM, Rustin GJ et al (2011) Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol 29:957–962
Zuniga A, Kakiashvili D, Jewett MA (2009) Surveillance in stage I nonseminomatous germ cell tumours of the testis. BJU Int 104:1351–1356
Kollmannsberger C, Moore C, Chi KN, Murray N et al (2010) Non-risk-adapted surveillance for patients with stage I nonseminomatous testicular germ-cell tumors: diminishing treatment-related morbidity while maintaining efficacy. Ann Oncol 21:1296–1301
Kollmannsberger C, Tyldesley S, Moore C et al (2011) Evolution in management of testicular seminoma: population-based outcomes with selective utilization of active therapies. Ann Oncol 22:808–814
Sturgeon JF, Moore MJ, Kakiashvili DM et al (2011) Non-risk-adapted surveillance in clinical stage I nonseminomatous germ cell tumors: the Princess Margaret Hospital’s experience. Eur Urol 59:556–562
Hao D, Seidel J, Brant R et al (1998) Compliance of clinical stage I non-seminomatous germ cell tumor patients with surveillance. J Urol 160:768–771
Acknowledgments
The work was partly supported by the Smoking Research Foundation, the Takeda Science Foundation, and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Conflict of interest
Yuasa T. received remuneration for a lecture from Pfizer Japan (Tokyo, Japan) and Novartis Pharma Japan (Tokyo, Japan). The other authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yuasa, T., Inoshita, N., Tanaka, H. et al. Surveillance policy for Japanese patients with stage I testicular germ cell cancer in the multi-detector computed tomography era. Int J Clin Oncol 20, 1198–1202 (2015). https://doi.org/10.1007/s10147-015-0828-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0828-1