Abstract
Background
Based on the results of the SPIRITS trial, combination chemotherapy of S-1 plus cisplatin (SP) is now considered the standard treatment for patients with advanced gastric cancer (AGC) in Japan. On the other hand, several non-Japanese studies have shown the efficacy of capecitabine plus cisplatin (XP), which has been used as the reference arm in recent global studies of AGC.
Methods
We retrospectively compared the efficacy and safety of SP and XP in first-line treatment for patients with AGC.
Results
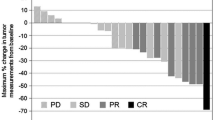
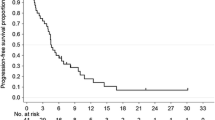
From August 2006 to November 2008, 26 AGC patients received XP in the context of 2 global trials (AVAGAST and ToGA), and 50 patients received SP during the same period. The objective response rate was 43.2 % in the SP group and 50 % in the XP group, with no significant difference (p = 0.62). There were also no significant differences in progression-free survival (median 5.8 vs. 5.2 months; p = 0.91) and overall survival (median 13.8 vs. 13.5 months; p = 0.97) between the SP and XP groups. The frequencies of hematological toxicities of grade 3 or more and non-hematological toxicities were not significantly different between the 2 groups. Although grade 1 or 2 hand–foot syndrome was more common in the XP group, no patients experienced grade 3 or more.
Conclusions
Although the retrospective nature of this study and the small number of patients is a major limitation, SP and XP were associated with similar efficacy and safety in patients with AGC.



Similar content being viewed by others
References
International Agency for Research on Cancer; GLOBOCAN 2008: http://www-dep.iarc.fr/CancerMondial.htm
Van Cutsem E, Moiseyenko VM, Tjulandin S et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24:4991–4997
Cunningham D, Starling N, Rao S et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36–46
Koizumi W, Narahara H, Hara T et al (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Kang YK, Kang WK, Shin DB et al (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 20:666–673
Ajani JA, Rodriguez W, Bodoky G et al (2010) Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 28:1547–1553
Sakata Y, Ohtsu A, Horikoshi N et al (1998) Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 34:1715–1720
Koizumi W, Kurihara M, Nakano S et al (2000) Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology 58:191–197
Boku N, Yamamoto S, Fukuda H et al (2009) Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 10:1063–1069
Okines AF, Norman AR, McCloud P et al (2009) Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol 20:1529–1534
National Comprehensive Cancer Network : NCCN clinical practice guidelines in Oncology, Gastric Cancer V.2.2011. (http://www.nccn.org/professionals/physician_gls/f_guidelines.asp)
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Ohtsu A, Shah MA, Van Cutsem E et al (2011) Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clinical Oncol 29:3968–3976. doi:10.1200/JCO.2011.36.2236
Ajani JA, Faust J, Ikeda K et al (2005) Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol 23:6957–6965
Daigo S, Takahashi Y, Fujieda M et al (2002) A novel mutant allele of the CYP2A6 gene (CYP2A6*11) found in a cancer patient who showed poor metabolic phenotype towards tegafur. Pharmacogenetics 12:299–306
Goh BC, Soo RA, Lim S et al (2008) Inter-ethnic variability of S-1 pharmacokinetics (PK) and correlation with CYP2A6 phenotyping. J Clin Oncol 26:113s (Abstr 2507)
Sakamoto J, Chin K, Kondo K et al (2006) Phase II study of a 4-week capecitabine regimen in advanced or recurrent gastric cancer. Anticancer Drugs 17:231–236
Lee JL, Kang YK, Kang HJ et al (2008) A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer 99:584–590
Yamada Y, Yamamoto S, Ohtsu A et al (2009) Impact of dihydropyrimidine dehydrogenase status of biopsy specimens on efficacy of irinotecan plus cisplatin, S-1, or 5-FU as first-line treatment of advanced gastric cancer patients in JCOG9912. J Clin Oncol 27:15s (Abstr 4535)
Ichikawa W, Takahashi T, Suto K et al (2004) Gene expressions for thymidylate synthase (TS), orotate phosphoribosyltransferase (OPRT), and thymidine phosphorylase (TP), not dihydropyrimidine dehydrogenase (DPD), influence outcome of patients (pts) treated with S-1 for gastric cancer (GC). J Clin Oncol (Meeting Abstracts) 2:4050
Napieralski R, Ott K, Kremer M et al (2005) Combined GADD45A and thymidine phosphorylase expression levels predict response and survival of neoadjuvant-treated gastric cancer patients. Clin Cancer Res 11:3025–3031
Salonga D, Danenberg KD, Johnson M et al (2000) Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res 6:1322–1327
Koizumi W, Okayasu I, Hyodo I et al (2008) Prediction of the effect of capecitabine in gastric cancer by immunohistochemical staining of thymidine phosphorylase and dihydropyrimidine dehydrogenase. Anticancer Drugs 19:819–824
Koizumi W, Saigenji K, Nakamaru N et al (1999) Prediction of response to 5′-deoxy-5-fluorouridine (5′-DFUR) in patients with inoperable advanced gastric cancer by immunostaining of thymidine phosphorylase/platelet-derived endothelial cell growth factor. Oncology 56:215–222
Takiguchi N, Ishii R, Koda K et al (2003) Thymidine phosphorylase expression correlates with malignant potential and anti-tumor effect of doxifluridine on gastric cancer: multivariate analysis for adjuvant chemotherapy doxifluridine vs. 5-fluorouracil. Oncol Rep 10:1105–1111
Meropol NJ, Gold PJ, Diasio RB et al (2006) Thymidine phosphorylase expression is associated with response to capecitabine plus irinotecan in patients with metastatic colorectal cancer. J Clin Oncol 24:4069–4077
Conflict of interest
None of the authors have financial or personal conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Shitara, K., Sawaki, A., Matsuo, K. et al. A retrospective comparison of S-1 plus cisplatin and capecitabine plus cisplatin for patients with advanced or recurrent gastric cancer. Int J Clin Oncol 18, 539–546 (2013). https://doi.org/10.1007/s10147-012-0416-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-012-0416-6