Abstract
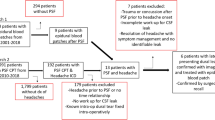
Cranioplasty is important for improving cosmesis and functional recovery after decompressive craniectomy. We assessed the incidence and predictors of post-cranioplasty epidural hematomas requiring surgical evacuation. A single-institution, retrospective study enrolled 194 consecutive patients who underwent a cranioplasty using custom-made hydroxyapatite between February 2008 and April 2022. Variables associated with postoperative epidural hematoma requiring surgical evacuation at the p < 0.1 level in unadjusted analysis were entered into multivariable analyses. Nine patients (4.6%) experienced postoperative epidural hematomas requiring evacuation, with time interval between craniectomy and cranioplasty <6 months (adjusted odds ratio (aOR), 20.75, p = 0.047), cranioplasty-to-bone shift > half of the bone thickness (aOR, 17.53, p = 0.008), >10 mm difference between pre-cranioplasty and post-cranioplasty midline brain shift contralateral to the cranioplasty (aOR, 17.26, p < 0.001), and non-resorbable duraplasty (aOR, 17.43, p = 0.011) identified as independent predictors. Seventeen patients (8.8%) experienced post-cranioplasty hydrocephalus requiring shunt placement. Twenty-six patients (13.4%) experienced postoperative infection. Sixteen patients (8.2%) had postoperative epileptic seizures. The identification of independent predictors of post-cranioplasty epidural hematomas requiring surgical evacuation will help identify at-risk patients, guide prophylactic care, and reduce morbidity of this common and important procedure.

Similar content being viewed by others
Data availability
We support data sharing within the restrictions of the ethical approval permissions. Requests can be made to the corresponding author.
References
Agner C, Dujovny M, Gaviria M (2002) Neurocognitive assessment before and after cranioplasty. Acta Neurochir (Wien) 144:1033–1040. https://doi.org/10.1007/s00701-002-0996-4
Honeybul S, Janzen C, Kruger K, Ho KM (2013) The impact of cranioplasty on neurological function. Br J Neurosurg 27:636–641. https://doi.org/10.3109/02688697.2013.817532
Parichay PJ, Khanapure K, Joshi KC, Aniruddha TJ, Sandhya M, Hegde AS (2017) Clinical and radiological assessment of cerebral hemodynamics after cranioplasty for decompressive craniectomy - a clinical study. J Clin Neurosci Off J Neurosurg Soc Australas 42:97–101. https://doi.org/10.1016/j.jocn.2017.04.005
Segal DH, Oppenheim JS, Murovic JA (1994) Neurological recovery after cranioplasty. Neurosurgery 34:729–731; discussion 731. https://doi.org/10.1227/00006123-199404000-00024
Song J, Liu M, Mo X, Du H, Huang H, Xu GZ (2014) Beneficial impact of early cranioplasty in patients with decompressive craniectomy: evidence from transcranial Doppler ultrasonography. Acta Neurochir (Wien) 156:193–198. https://doi.org/10.1007/s00701-013-1908-5
Andrabi SM, Sarmast AH, Kirmani AR, Bhat AR (2017) Cranioplasty: indications, procedures, and outcome - an institutional experience. Surg Neurol Int 8:91. https://doi.org/10.4103/sni.sni_45_17
Stiver SI, Wintermark M, Manley GT (2008) Reversible monoparesis following decompressive hemicraniectomy for traumatic brain injury. J Neurosurg 109:245–254. https://doi.org/10.3171/JNS/2008/109/8/0245
Bobinski L, Koskinen L-OD, Lindvall P (2013) Complications following cranioplasty using autologous bone or polymethylmethacrylate—retrospective experience from a single center. Clin Neurol Neurosurg 115:1788–1791. https://doi.org/10.1016/j.clineuro.2013.04.013
Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D (2010) Outcomes of cranial repair after craniectomy. J Neurosurg 112:1120–1124. https://doi.org/10.3171/2009.6.JNS09133
Krause-Titz UR, Warneke N, Freitag-Wolf S, Barth H, Mehdorn HM (2016) Factors influencing the outcome (GOS) in reconstructive cranioplasty. Neurosurg Rev 39:133–139. https://doi.org/10.1007/s10143-015-0678-3
Hardy H, Tollard E, Derrey S, Delcampe P, Péron J-M, Fréger P, Proust F (2012) Clinical and ossification outcome of custom-made hydroxyapatite prothese for large skull defect. Neurochirurgie 58:25–29. https://doi.org/10.1016/j.neuchi.2011.09.006
Lindner D, Schlothofer-Schumann K, Kern B-C, Marx O, Müns A, Meixensberger J (2017) Cranioplasty using custom-made hydroxyapatite versus titanium: a randomized clinical trial. J Neurosurg 126:175–183. https://doi.org/10.3171/2015.10.JNS151245
Stefini R, Zanotti B, Nataloni A, Martinetti R, Scafuto M, Colasurdo M, Tampieri A (2015) The efficacy of custom-made porous hydroxyapatite prostheses for cranioplasty: evaluation of postmarketing data on 2697 patients. J Appl Biomater Funct Mater 13:e136–e144. https://doi.org/10.5301/jabfm.5000211
Wind JJ, Ohaegbulam C, Iwamoto FM, Black PM, Park JK (2013) Immediate titanium mesh cranioplasty for treatment of postcraniotomy infections. World Neurosurg 79:207.e11–207.e13. https://doi.org/10.1016/j.wneu.2011.02.013
Zanotti B, Zingaretti N, Verlicchi A, Robiony M, Alfieri A, Parodi PC (2016) Cranioplasty: review of materials. J Craniofac Surg 27:2061–2072. https://doi.org/10.1097/SCS.0000000000003025
Still M, Kane A, Roux A, Zanello M, Dezamis E, Parraga E, Sauvageon X, Meder J-F, Pallud J (2018) Independent factors affecting postoperative complication rates after custom-made porous hydroxyapatite cranioplasty: a single-center review of 109 cases. World Neurosurg 114:e1232–e1244. https://doi.org/10.1016/j.wneu.2018.03.181
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative STROBE (2008) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61:344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008
Lee GS, Park SQ, Kim R, Cho SJ (2015) Unexpected severe cerebral edema after cranioplasty: case report and literature review. J Korean Neurosurg Soc 58:76. https://doi.org/10.3340/jkns.2015.58.1.76
Lillemäe K, Järviö JA, Silvasti-Lundell MK, Antinheimo JJ-P, Hernesniemi JA, Niemi TT (2017) Incidence of postoperative hematomas requiring surgical treatment in neurosurgery: a retrospective observational study. World Neurosurg 108:491–497. https://doi.org/10.1016/j.wneu.2017.09.007
Broughton E, Pobereskin L, Whitfield PC (2014) Seven years of cranioplasty in a regional neurosurgical centre. Br J Neurosurg 28:34–39. https://doi.org/10.3109/02688697.2013.815319
Lee L, Ker J, Quah BL, Chou N, Choy D, Yeo TT (2013) A retrospective analysis and review of an institution’s experience with the complications of cranioplasty. Br J Neurosurg 27:629–635. https://doi.org/10.3109/02688697.2013.815313
Zanaty M, Chalouhi N, Starke RM, Clark SW, Bovenzi CD, Saigh M, Schwartz E, Kunkel ESI, Efthimiadis-Budike AS, Jabbour P, Dalyai R, Rosenwasser RH, Tjoumakaris SI (2015) Complications following cranioplasty: incidence and predictors in 348 cases. J Neurosurg 123:182–188. https://doi.org/10.3171/2014.9.JNS14405
Schuss P, Vatter H, Marquardt G, Imöhl L, Ulrich CT, Seifert V, Güresir E (2012) Cranioplasty after decompressive craniectomy: the effect of timing on postoperative complications. J Neurotrauma 29:1090–1095. https://doi.org/10.1089/neu.2011.2176
Mukherjee S, Thakur B, Haq I, Hettige S, Martin AJ (2014) Complications of titanium cranioplasty—a retrospective analysis of 174 patients. Acta Neurochir (Wien) 156:989–998; discussion 998. https://doi.org/10.1007/s00701-014-2024-x
Hirschmann D, Kranawetter B, Kirchschlager C, Tomschik M, Wais J, Winter F, Millesi M, Herta J, Roessler K, Dorfer C (2021) Cranioplasty following ventriculoperitoneal shunting: lessons learned. Acta Neurochir (Wien) 163:441–446. https://doi.org/10.1007/s00701-020-04597-y
Malcolm JG, Rindler RS, Chu JK, Grossberg JA, Pradilla G, Ahmad FU (2016) Complications following cranioplasty and relationship to timing: a systematic review and meta-analysis. J Clin Neurosci 33:39–51. https://doi.org/10.1016/j.jocn.2016.04.017
Huang Y-H, Lee T-C, Chen W-F, Wang Y-M (2011) Safety of the nonabsorbable dural substitute in decompressive craniectomy for severe traumatic brain injury. J Trauma Inj Infect Crit Care 71:533–537. https://doi.org/10.1097/TA.0b013e318203208a
Raghavan A, Wright JM, Huang Wright C, Sajatovic M, Miller J (2018) Effect of dural substitute and technique on cranioplasty operative metrics: a systematic literature review. World Neurosurg 119:282–289. https://doi.org/10.1016/j.wneu.2018.08.024
Sun H, Wang H, Diao Y, Tu Y, Li X, Zhao W, Ren J, Zhang S (2018) Large retrospective study of artificial dura substitute in patients with traumatic brain injury undergo decompressive craniectomy. Brain Behav 8:e00907. https://doi.org/10.1002/brb3.907
Vakis A, Koutentakis D, Karabetsos D, Kalostos G (2006) Use of polytetrafluoroethylene dural substitute as adhesion preventive material during craniectomies. Clin Neurol Neurosurg 108:798–802. https://doi.org/10.1016/j.clineuro.2005.11.026
Klinger DR, Madden C, Beshay J, White J, Gambrell K, Rickert K (2014) Autologous and acrylic cranioplasty: a review of 10 years and 258 cases. World Neurosurg 82:e525–e530. https://doi.org/10.1016/j.wneu.2013.08.005
Roth J, Galeano E, Milla S, Hartmannsgruber MW, Weiner HL (2011) Multiple epidural hematomas and hemodynamic collapse caused by a subgaleal drain and suction-induced intracranial hypotension: case report. Neurosurgery 68:E271–E275; discussion E276. https://doi.org/10.1227/NEU.0b013e3181fe6165
Jeong SH, Wang US, Kim SW, Ha SW, Kim JK (2016) Symptomatic epidural fluid collection following cranioplasty after decompressive craniectomy for traumatic brain injury. Korean J Neurotrauma 12:6. https://doi.org/10.13004/kjnt.2016.12.1.6
Peng A, Qi W, Cao D, Zhao Y, Gao K, Cheng C, Wu Y (2018) Fibrinolytic therapy improves outcomes in patients with epidural hematomas following cranioplasty: a pilot study. J Neurol Surg Part Cent Eur Neurosurg 79:039–044. https://doi.org/10.1055/s-0037-1606848
Author information
Authors and Affiliations
Contributions
A.B., M.S., and J.P. wrote the main manuscript text. A.E. and J.P. made the statistical analyses. J.P. prepared Fig. 1. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
An institutional review board approved the study protocol (IRB00011687, IRB#1:2022/41). The requirement to obtain informed consent was waived according to French legislation.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marc Zanello and Johan Pallud participated equally.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bedioui, A., Elia, A., Still, M. et al. Predictors of postoperative epidural hematomas after custom-made porous hydroxyapatite cranioplasty: a single-center experience of 194 consecutive cases. Neurosurg Rev 46, 132 (2023). https://doi.org/10.1007/s10143-023-02039-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02039-8