Abstract
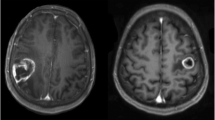
The role of surgery on central area metastasis remains unclear, and outcome data are still controversial. The aim of our study is to analyze the predictive value of clinical and surgical data on motor and functional outcome of patients, taking into account new emerging data on boundary irregularity of brain metastasis. We retrospectively analyzed 47 consecutive patients who underwent surgery assisted by neurophysiologic monitoring for a solitary metastasis in central area between 2010 and 2013. Inclusion criteria were as follows: good functional status (Karnofsky Performance Status (KPS) ≥70), controlled systemic disease, and absence of extra-cranial dissemination. At 1-month follow up, motor and functional outcomes were compared with preoperative clinical status, response to corticosteroids, extent of tumor resection, boundary irregularity, and size of tumor. Gross total resection was achieved in 93.6 % of cases. In preoperative symptomatic patients, motor outcome (according to Medical Research Council grading scale) improved in 55.5 % and worsened in 16.7 %, while functional outcome (according to KPS score) improved in 50 % and worsened in 14.2 % of cases. No worsening occurred in preoperative asymptomatic patients. Motor outcome resulted to be not correlated with preoperative deficits, tumor volume, or preoperative response to corticosteroid treatment. Remarkably, motor outcome and extent of surgical resection appeared strongly correlated with tumor boundary irregularity (p < 0.05). Surgery with neurophysiologic monitoring on motor area metastasis can improve functional and motor condition in selected patients. Tumor volume does not represent a limit in surgery. The high correlation between clinical outcome, resection rate, and tumor boundary irregularity strengthens a new belief on the infiltrative growing pattern of brain metastasis. Motor function was evaluated according to Medical Research Council grading scale (Ott et al. 2014) while functional status was assessed according to KPS score.


Similar content being viewed by others
References
Arita H, Narita Y, Miyakita Y, Ohno M, Sumi M, Shibui S (2013) Risk factors for early death after surgery in patients with brain metastases: reevaluation of the indications for and role of surgery. J Neurooncol 116(1):145–152. doi:10.1007/s11060-013-1273-5
Berghoff AS, Rajky O, Winkler F, Bartsch R, Furtner J, Hainfellner JA, Goodman SL, Weller M, Schittenhelm J, Preusser M (2013) Invasion patterns in brain metastases of solid cancers. Neuro Oncol 15(12):1664–1672. doi:10.1093/neuonc/not112
Cedzich C, Taniguchi M, Schäfer S, Schramm J (1996) Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery 38(5):962–970
Della Puppa A, De Pellegrin S, d’Avella E, Gioffrè G, Rossetto M, Gerardi A, Lombardi G, Manara R, Munari M, Saladini M, Scienza R (2013) 5-aminolevulinic acid (5-ALA) fluorescence guided surgery of high-grade gliomas in eloquent areas assisted by functional mapping. our experience and review of the literature. Acta Neurochir (Wien) 155(6):965–972. doi:10.1007/s00701-013-1660-x
Duffau H (2001) Recovery from complete emiplegia following resection of a retrocentral metastasis: the prognostic value of intraoperative cortical stimulation. J Neurosurg 95(6):1050–1052
Karnofsky DA, Burchenal JH (1949) The Clinical Evaluation of Chemotherapeutic Agents in Cancer. In: MacLeod CM (Ed), Evaluation of Chemotherapeutic Agents. Columbia Univ Press, pp 196
Krieg SM, Schäffner M, Shiban E, Droese D, Obermüller T, Gempt J, Meyer B, Ringel F (2013) Reliability of intraoperative neurophysiological monitoring using motor evoked potentials during resection of metastases in motor-eloquent brain regions: clinical article. J Neurosurg 118(6):1269–1278. doi:10.3171/2013.2.JNS121752
Luther N, Kondziolka D, Kano H, Mousavi SH, Flickinger JC, Lunsford LD (2013) Motor function after stereotactic radiosurgery for brain metastases in the region of the motor cortex. J Neurosurg 119(3):683–688. doi:10.3171/2013.6.JNS122081
Neuloh G, Pechstein U, Schramm J (2007) Motor tract monitoring during insular glioma surgery. J Neurosurg 106(4):582–592
Nieder C, Astner ST, Andratschke NH, Marienhagen K (2011) Postoperative treatment and prognosis of patients with resected single brain metastasis: How useful are established prognostic scores? Clin Neurol Neurosurg 113(2):98–103. doi:10.1016/j.clineuro.2010.09.009
Obermueller T, Schaeffner M, Gerhardt J, Meyer B, Ringel F, Krieg SM (2014) Risks of postoperative paresis in motor eloquently and non-eloquently located brain metastases. BMC Cancer 14:14–21. doi:10.1186/1471-2407-14-21
Ott C, Kerscher C, Luerding R, Doenitz C, Hoehne J, Zech N, Seemann M, Schlaier J, Brawanski A (2014) The Impact of Sedation on Brain Mapping: A Prospective, Interdisciplinary, Clinical Trial. Neurosurgery 7. doi:10.1227/NEU.0000000000000359
Paternostro-Sluga T, Grim-Stieger M, Posch M, Schuhfried O, Vacariu G, Mittermaier C, Bittner C, Fialka-Moser V (2008) Reliability and validity of the medical research council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med 40(8):665–671. doi:10.2340/16501977-0235
Rowed DW, Houlden DA, Basavakumar DG (1997) Somatosensory evoked potential identification of sensorimotor cortex in removal of intracranial neoplasm. Can J Neurol Sci 24(2):116–120
Sanai N, Berger MS (2010) Intraoperative stimulation techniques for functional pathway preservation and glioma resection. Neurosurg Focus 28(2), E1. doi:10.3171/2009.12.FOCUS09266
Seidel K, Beck J, Stieglitz L, Schucht P, Raabe A (2013) The warning-sign hierarchy between quantitative subcortical motor mapping and continuous motor evoked potential monitoring during resection of supratentorial brain tumors. J Neurosurg 118(2):287–296. doi:10.3171/2012-10-JNS12895
Shinoura N, Yoshida M, Yamada R, Tabei Y, Saito K, Suzuki Y, Takayama Y, Yagi K (2009) Awake surgery with contiunuous motor testing for resection of brain tumors in the primary motor area. J Clin Neurosci 16(2):188–194. doi:10.1016/j.jocn.2008.02.013
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30(4):419–425. doi:10.1200/JCO.2011.38.0527
Tan TC, McL Black P (2003) Image-guided craniotomy for cerebral metastases: techniques and outcomes. Neurosurgery 53(1):82–89
Walter J, Kuhn SA, Waschke A, Kalff R, Ewald C (2011) Operative treatment of subcortical metastatic tumours in the central region. J Neurooncol 103(3):567–573. doi:10.1007/s11060-010-0420-5
Weil RJ, Lonser RR (2005) Selective excision of metastatic brain tumors originating in the motor cortex with preservation of function. J Clin Oncol 23(6):1209–1217
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Yavor Enchev, Varna, Bulgaria
The authors retrospectively analyzed their own series of 47 consecutive patients with solitary metastatic lesions in motor area. The patients were included in the study based on strict enrollment criteria (KPS ≥70, absence of extracranial metastases and controlled systemic disease) and the tumours were resected with the assistance of neuronavigation and neurophysiologic monitoring. The motor and functional surgical outcome were evaluated and compared with the preoperative state. Based on their results, between their mostly confirmative data, the authors noted something very interesting, namely that the motor outcome and the extent of tumour resection strongly correlated with the so-called “tumour margins irregularity”. By my opinion, further studies focused on defining the criteria for tumour margins irregularity, evaluating its degree of expression and eventually its potential prognostic value for the extent of surgical resection and motor outcome, are recommended.
Fumio Yamaguchi, Tokyo, Japan
Rossetto et al. reported the utilization of brain mapping technique in the removal of metastatic brain tumors. As a nature of metastatic tumors, boundaries are macroscopically clear in most cases and easy to remove without damaging adjacent neural structure. However, as the authors discussed, it is difficult to remove the tumor in case of irregular tumor margin, resulting in subtotal removal or complete removal with postoperative neurological deteriorations in eloquent areas. If the invaded brain tissue includes any eloquent fibers, the decision of removal extent must be carefully made according to intraoperative brain mapping. Also, resection of the surrounding white matter is important to prevent local recurrence. Based on this concept, tractography integrated in neuronavigation system in conjunction with intraoperative brain mapping should be used routinely in case of tumors in eloquent brain even if tumor boundaries are clear. The resection of metastatic brain tumor may be considered simple and relatively easy surgery, however, the prevention of postoperative neurological deterioration should be set high value on for patients who have limited remaining days. From this point of view intraoperative mapping is very important and essential technique.
Rights and permissions
About this article
Cite this article
Rossetto, M., Ciccarino, P., Lombardi, G. et al. Surgery on motor area metastasis. Neurosurg Rev 39, 71–78 (2016). https://doi.org/10.1007/s10143-015-0648-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-015-0648-9