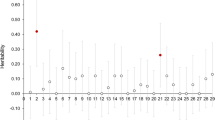
Abstract
The ability to identify sex is necessary in population biology for a proper understanding of the dynamics of a population. In Atlantic halibut, phenotypic sex identification is not possible due to the lack of significant external morphological differences. We developed an Illumina SNP panel for Atlantic halibut with 4000 SNPs spread evenly throughout the genome with a minor allele frequency MAF ≥ 0.4, except for N = 249 SNPs located in a sex-determining region on chromosome 12, N = 176 of these SNPs were selected to genetically identify male and female individuals using a DAPC analysis. The genomic identification of sex allows for non-lethal sex determination and validation of sex identification in the field. The SNP panel is a new genomic resource for Atlantic halibut that will make it possible to generate the genotypic data for the large number of individuals needed to estimate population abundance using genomics and the Close Kin Mark Recapture (CKMR) approach, an emerging component of fisheries management and stock monitoring.




Similar content being viewed by others
Data Availability
Data analyzed in this study will be made available in GenBank (NCBI) and scripts related to data analysis will be uploaded to GitHub at https://github.com/weiseell/ForTheHalibut, both at acceptance for publication.
References
Andrews KR et al (2016) Harnessing the power of RADseq for ecological and evolutionary genomics. Nat Rev Genet 17(2):81–92
Baird NA et al (2008) Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS One 3:e3376
Bravington MV, Grewe PM, Davies CR (2016) Absolute abundance of southern bluefin tuna estimated by close-kin mark-recapture. Nat Commun 7:1–8
Capel B (2017) Vertebrate sex determination: evolutionary plasticity of a fundamental switch. Nat Rev Genet 18:675–689
Cargnelli LM, Griesbach SJ, Morse WW (1999) NOAA technical memorandum NMFS. Atlantic halibut, Hippoglossus hippoglossus, life history and habitat characteristics
Cerviño S (2014) Estimating growth from sex ratio-at-length data in species with sexual size dimorphism. Fish Res 160:112–119
Chen S et al (2014) Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat Genet 46:253–260
Cox SP, Benson A, den Heyer CE (2016) Framework for the assessment of Atlantic halibut stocks on Scotian Shelf and Southern Grand Banks. DFO. Canadian Science Advisory Secretariat
DFO (2019) Stock assessment of Gulf of ST. Lawrence (4RST) Atlantic halibut in 2018. DFO. Canadian Science Advisory Secretariat Science Response
DFO (2020) Stock status update of Atlantic halibut (Hippoglossus hippoglossus) on the Scotian Shelf and Southern Grand Banks in NAFO divisions 3NOPS4VWX5ZC. DFO. Canadian Science Advisory Secretariat Science Response
Edvardsen RB et al (2022) Heterochiasmy and the establishment of gsdf as a novel sex determining gene in Atlantic halibut. PLoS Genet 18:e1010011
Einfeldt AL et al (2021) Chromosome level reference of Atlantic halibut Hippoglossus hippoglossus provides insight into the evolution of sexual determination systems. Mol Ecol Resour 21:1686–1696
Ferchaud AL et al (2022) Chromosome-level assembly reveals a putative Y-autosomal fusion in the sex determination system of the Greenland halibut (Reinhardtius hippoglossoides). G3-Genes Genom Genet 12(1)
Furman BLS et al (2020) Sex chromosome evolution: so many exceptions to the rules. Genome Biol Evol 12:750–763
Grasso GM (2008) What appeared limitless plenty: the rise and fall of the nineteenth-century Atlantic halibut fishery. Environ Hist 13:66–91
Guerrero-Cózar I et al (2021) Chromosome anchoring in Senegalese sole (Solea senegalensis) reveals sex-associated markers and genome rearrangements in flatfish. Sci Rep 11(1):1–16
Hughes V, Benfey TJ, Martin-Robichaud DJ (2008) Effect of rearing temperature on sex ratio in juvenile Atlantic halibut, Hippoglossus hippoglossus. Environ Biol Fishes 81:415–419
Jasonowicz AJ et al (2022) Generation of a chromosome-level genome assembly for Pacific halibut (Hippoglossus stenolepis) and characterization of its sex-determining genomic region. Mol Ecol Resour 22(7):2685–2700. https://doi.org/10.1111/1755-0998.13641
Jombart T, Alex B (2008) Genetics and population analysis adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24(11):1403–1405
Karlsson S et al (2011) Generic genetic differences between farmed and wild Atlantic salmon identified from a 7K SNP-chip. Mol Ecol Resour 11:247–253
Kess T et al (2021) A putative structural variant and environmental variation associated with genomic divergence across the Northwest Atlantic in Atlantic halibut. ICES J Mar Sci 78:2371–2384
Kurtis Trzcinski M, Don W, Bowen. (2016) The recovery of Atlantic halibut: a large, long-lived, and exploited marine predator. ICES J Mar Sci 73:1104–1114
McCracken FD (1958) On the biology and fishery of the Canadian Atlantic halibut, Hippoglossus hippoglossus L. J Fish Res Board Can 15:1269–1311
Myosho T, Takehana Y, Hamaguchi S, Sakaizumi M (2015) Turnover of sex chromosomes in celebensis group medaka fishes. G3-Genes Genom Genet 5(12):2685–2691
van Nes S, Andersen Ø (2006) Temperature effects on sex determination and ontogenetic gene expression of the aromatases Cyp19a and Cyp19b, and the estrogen receptors Esr1 and Esr2 in Atlantic halibut (Hippoglossus hippoglossus). Mol Reprod Dev 73:1481–1490
Nunziata SO, Weisrock DW (2018) Estimation of contemporary effective population size and population declines using RAD sequence data. Heredity 120:196–207
Ruzzante DE et al (2019) Validation of close-kin mark–recapture (CKMR) methods for estimating population abundance. Methods Ecol Evol 10:1445–1453
Santure AW et al (2010) On the use of large marker panels to estimate inbreeding and relatedness: empirical and simulation studies of a pedigreed zebra finch population typed at 771 SNPs. Mol Ecol 19:1439–1451
Semba Y (2018) Significance of sex-specific ecological and life history traits on the sustainable exploitation of sharks. In: Ichiro A, Takashi Y, Akinori T (eds) Fish population dynamics, monitoring, and management. Springer, Tokyo, pp 77–104 (November 30, 2022)
Shackell NL et al (2021) Spatial ecology of Atlantic halibut across the Northwest Atlantic: a recovering species in an era of climate change. Rev Fish Sci Aquac 30(3):281–305
Wright AE et al (2017) Convergent recombination suppression suggests role of sexual selection in guppy sex chromosome formation. Nat Commun 8(1):1–10
Yáñez JM et al (2020) High-throughput single nucleotide polymorphism (SNP) discovery and validation through whole-genome resequencing in Nile tilapia (Oreochromis niloticus). Mar Biotechnol 22:109–117
Acknowledgements
We thank Danni Harper of DFO, Albert Moore and Dylan Buchanan of Javitech Inc., and the vast number of fisheries observers who contributed and continue to contribute to the sample collection required for this project.
Funding
This research (microarray development and manufacture) was funded by a grant from Fisheries and Oceans Canada (DFO) program: Sustainable Fisheries Science Fund (SFSF) for a project entitled: “Genomics and the estimation of population abundance in Atlantic halibut (Hippoglossus hippoglossus) using the ‘Close Kin Mark Recapture’ framework.” Funding for genotyping comes from the Government of Canada and the province of Newfoundland and Labrador’s joint Atlantic Fisheries Fund’s support of the Centre for Fisheries Ecosystems Research (Fisheries and Marine Institute of Memorial University of Newfoundland) the objectives of which are to develop new and improved groundfish stock assessment models and methods. The study is part of EMWs PhD research. EMW is funded by the Fisheries and Oceans grant, a Nova Scotia graduate student scholarship and Dalhousie University funds and a Natural Sciences and Engineering Research Council of Canada Discovery grant to DER. We acknowledge NSERC Discovery Grants to DER and JMF.
Author information
Authors and Affiliations
Contributions
The microarray was designed by Mallory Van Wyngaarden, Tony Kess, Anthony L. Einfeldt, and Daniel E. Ruzzante. Ellen M. Weise, Mallory Van Wyngaarden, Tony Kess, and Anthony L. Einfeldt performed the analysis. Daniel E. Ruzzante, Cornelia Den Heyer, Joanna Mills Flemming, and Jonathan Fisher designed the CKMR project and Reina Ditta and Guillaume Pare contributed new tools. Ellen M. Weise, Mallory Van Wyngaarden, and Daniel E. Ruzzante wrote the initial draft. All authors commented and edited the paper.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Weise, E.M., Van Wyngaarden, M., Den Heyer, C. et al. SNP Panel and Genomic Sex Identification in Atlantic Halibut (Hippoglossus hippoglossus). Mar Biotechnol 25, 580–587 (2023). https://doi.org/10.1007/s10126-023-10227-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-023-10227-2