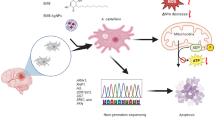
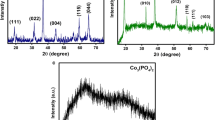
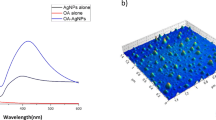
Abstract
Acanthamoeba are free living amoebae that are the causative agent of keratitis and granulomatous amoebic encephalitis. Alpha-Mangostin (AMS) is a significant xanthone; that demonstrates a wide range of biological activities. Here, the anti-amoebic activity of α-Mangostin and its silver nano conjugates (AMS-AgNPs) were evaluated against pathogenic A. castellanii trophozoites and cysts in vitro. Amoebicidal assays showed that both AMS and AMS-AgNPs inhibited the viability of A. castellanii dose-dependently, with an IC50 of 88.5 ± 2.04 and 20.2 ± 2.17 μM, respectively. Both formulations inhibited A. castellanii-mediated human keratinocyte cell cytopathogenicity. Functional assays showed that both samples caused apoptosis through the mitochondrial pathway and reduced mitochondrial membrane potential and ATP production, while increasing reactive oxygen species (ROS) and nicotinamide adenine dinucleotide phosphate (NADPH) cytochrome-c reductase in the cytosol. Whole transcriptome sequencing of A. castellanii showed the expression of 826 genes, with 447 genes being up-regulated and 379 genes being down-regulated post treatment. The Kyoto Encyclopedia of Genes and Genomes analysis showed that the majority of genes were linked to apoptosis, autophagy, RAP1, AGE-RAGE and oxytocin signalling pathways. Seven genes (PTEN, H3, ARIH1, SDR16C5, PFN, glnA GLUL, and SRX1) were identified as the most significant (Log2 (FC) value 4) for molecular mode of action in vitro. Future in vivo studies with AMS and nanoconjugates are needed to realize the clinical potential of this work.
Graphical abstract










Similar content being viewed by others
Data availability
The data are available upon request to the author.
References
Ahmed U, Anwar A, Ong SK, Anwar A, Khan NA (2022a) Applications of medicinal chemistry for drug discovery against Acanthamoeba infections. Med Res Rev 42(1):462–512
Ahmed U, Ho KY, Simon SE, Saad SM, Ong SK, Anwar A, Tan KO, Sridewi N, Khan KM, Khan NA, Anwar A (2022b) Potential anti-acanthamoebic effects through inhibition of CYP51 by novel quinazolinones. Acta Trop 231:106440
Ahmed U, Manzoor M, Qureshi S, Mazhar M, Fatima A, Aurangzeb S, Hamid M, Khan KM, Khan NA, Rashid Y, Anwar A, (2023) Anti-amoebic effects of synthetic acridine-9 (10H)-one against brain-eating amoebae. Acta Trop 239:106824. https://doi.org/10.1016/j.actatropica.2023.106824
Al-Massarani SM, El Gamal AA, Al-Musayeib NM, Mothana RA, Basudan OA, Al-Rehaily AJ, Farag M, Assaf MH, El Tahir KH, Maes L (2013) Phytochemical, antimicrobial and antiprotozoal evaluation of Garcinia mangostana pericarp and α-mangostin, its major xanthone derivative. Molecule 18(9):10599–10608
Anwar A, Khan NA, Siddiqui R (2018) Combating Acanthamoeba spp. cysts: what are the options? Parasit Vector 11(1):1–6
Anwar A, Ting EL, Anwar A, ul Ain N, Faizi S, Shah MR, Khan NA, Siddiqui R (2020) Antiamoebic activity of plant-based natural products and their conjugated silver nanoparticles against Acanthamoeba castellanii (ATCC 50492). AMB Express 10(1):1–10
Anwar A, Siddiqui R, Hussain MA, Ahmed D, Shah MR, Khan NA (2018b) Silver nanoparticle conjugation affects antiacanthamoebic activities of amphotericin B, nystatin, and fluconazole. Parasitol Res 117:265–271
Anwar A, Numan A, Siddiqui R, Khalid M, Khan NA (2019) Cobalt nanoparticles as novel nanotherapeutics against Acanthamoeba castellanii. Parasit Vector 12(1):1–10
Baek MK, Kim MK, Cho HJ, Lee JA, Yu J, Chung HE, Choi SJ (2011) Factors influencing the cytotoxicity of zinc oxide nanoparticles: particle size and surface charge. J Phys: Conf Ser 304(1):012044
Baig AM (2015) Pathogenesis of amoebic encephalitis: are the amoebae being credited to an ‘inside job’done by the host immune response? Acta Trop 148:72–76
Chuprom J, Sangkanu S, Mitsuwan W, Boonhok R, Mahabusarakam W, Singh LR, Dumkliang E, Jitrangsri K, Paul AK, Surinkaew S, Wilairatana P (2022) Anti-Acanthamoeba activity of a semi-synthetic mangostin derivative and its ability in removal of Acanthamoeba triangularis WU19001 on contact lens. PeerJ 10:e14468
Cully M, You H, Levine AJ, Mak TW (2006) Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Canc 6(3):184–192
Damhorst GL, Watts A, Hernandez-Romieu A, Mel N, Palmore M, Ali IK, Neill SG, Kalapila A, Cope JR (2022) Acanthamoeba castellanii encephalitis in a patient with AIDS: a case report and literature review. Lanc Infect Dis 22(2):e59-65
Davey RJ, Moens PD (2020) Profilin: many facets of a small protein. Biophys Rev 12(4):827–849
Djoko KY, Phan MD, Peters KM, Walker MJ, Schembri MA, McEwan AG (2017) Interplay between tolerance mechanisms to copper and acid stress in Escherichia coli. Proc Natl Acad Sci 114 (26):6818-23
Ebert B, Kisiela M, Maser E (2016) Transcriptional regulation of human and murine short-chain dehydrogenase/reductases (SDRs)–an in silico approach. Drug Metab Rev 48(2):183–217
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516
Fang Y, Su T, Qiu X, Mao P, Xu Y, Hu Z, Zhang Y, Zheng X, Xie P, Liu Q (2016) Protective effect of alpha-mangostin against oxidative stress induced-retinal cell death. Sci Rep 6(1):1–5
Fleury C, Mignotte B, Vayssiere JL (2002) Mitochondrial reactive oxygen species in cell death signaling. Biochim 84:131–141
Georgescu MM (2010) PTEN tumor suppressor network in PI3K-Akt pathway control. Gene Canc 1(12):1170–1177
González-Robles A, Salazar-Villatoro L, Omaña-Molina M, Reyes-Batlle M, Martín-Navarro CM, Lorenzo-Morales J (2014) Morphological features and in-vitro cytopathic effect of Acanthamoeba griffini trophozoites isolated from a clinical case. J Parasitol Res 256310:2014
Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Gene Dev 13:1899–1911
Gutiérrez-Escobedo G, Hernández-Carreón O, Morales-Rojano B, Revuelta-Rodríguez B, Vázquez-Franco N, Castaño I, De Las Peñas A (2020) Candida glabrata peroxiredoxins, Tsa1 and Tsa2, and sulfiredoxin, Srx1, protect against oxidative damage and are necessary for virulence. Fung Gen Biol 135:103287
Gutierrez-Orozco F, Chitchumroonchokchai C, Lesinski GB, Suksamrarn S, Failla ML (2013) α-Mangostin: anti-inflammatory activity and metabolism by human cells. J Agric Food Chem 61(16):3891–3900
Herrera-Aco DR, Medina-Campos ON, Pedraza-Chaverri J, Sciutto-Conde E, Rosas-Salgado G, Fragoso-González G (2019) Alpha-mangostin: anti-inflammatory and antioxidant effects on established collagen-induced arthritis in DBA/1J mice. Food Chem Toxicol 124:300–315
Hsieh SC, Huang MH, Cheng CW, Hung JH, Yang SF, Hsieh YH (2013) α-Mangostin induces mitochondrial dependent apoptosis in human hepatoma SK-Hep-1 cells through inhibition of p 38 MAPK pathway. Apop 18:1548–1560
Ibrahim MY, Hashim NM, Mariod AA, Mohan S, Abdulla MA, Abdelwahab SI, Arbab IA (2016) α-Mangostin from Garcinia mangostana Linn: an updated review of its pharmacological properties. Arab J Chem 9(3):317–329
Jeyamogan S, Khan NA, Anwar A, Shah MR, Siddiqui R (2018) Cytotoxic effects of Benzodioxane, Naphthalene diimide, Porphyrin and Acetamol derivatives on HeLa cells. SAGE Open Med 6:2050312118781962
Jha BK, Jung HJ, Seo I, Suh SI, Suh MH, Baek WK (2015) Juglone induces cell death of Acanthamoeba through increased production of reactive oxygen species. Exp Parasitol 159:100–106
Joshi DC, Bakowska JC (2011) Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. J vis Exp 23(51):e2704
Kaomongkolgit R, Jamdee K, Pumklin J, Pavasant P (2013) Laboratory evaluation of the antibacterial and cytotoxic effect of alpha-mangostin when used as a root canal irrigant. Indian J Dent 4(1):12–17
Khan NA, Anwar A, Siddiqui R (2019) Acanthamoeba keratitis: current status and urgent research priorities. Curr Med Chem 26(30):5711–5726
Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Method 12(4):357–360
Koutsogiannis Z, MacLeod ET, Maciver SK (2019) G418 induces programmed cell death in Acanthamoeba through the elevation of intracellular calcium and cytochrome c translocation. Parasitol Res 118(2):641–651
Krishnan K, Moens PD (2009) Structure and functions of profilins. Biophys Rev 1(2):71–81
Li L, Brunner I, Han AR, Hamburger M, Kinghorn AD, Frye R, Butterweck V (2011) Pharmacokinetics of α-mangostin in rats after intravenous and oral application. Mol Nutri Food Res 55(S1):S67-74
Lockman PR, Koziara JM, Mumper RJ, Allen DD (2004) Nanoparticle surface charges alter blood–brain barrier integrity and permeability. J Drug Target 12(9–10):635–641
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genom Biol 15(12):1–21
Loyola A, Almouzni G (2007) Marking histone H3 variants: how, when and why? Trend Biochem Sci 32(9):425–433
Marciano-Cabral F, Cabral G (2003) Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 16(2):273–307
Márquez-Valadez B, Lugo-Huitrón R, Valdivia-Cerda V, Miranda-Ramírez LR, Pérez-De La Cruz V, González-Cuahutencos O, Rivero-Cruz I, Mata R, Santamaría A, Pedraza-Chaverrí J (2009) The natural xanthone α-mangostin reduces oxidative damage in rat brain tissue. Nutr Neurosci 12(1):35–42
Masri A, Khan NA, Zoqratt MZ, Ayub Q, Anwar A, Rao K, Shah MR, Siddiqui R (2021) Transcriptome analysis of Escherichia coli K1 after therapy with hesperidin conjugated with silver nanoparticles. BMC Microbiol 21(1):1–1
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Method 5(7):621–628
Mungroo MR, Khan NA, Anwar A, Siddiqui R (2022) Nanovehicles in the improved treatment of infections due to brain-eating amoebae. Int Microbiol 25(2):225–235. https://doi.org/10.1007/s10123-021-00201-0
Myung J, Kim KB, Crews CM (2001) The ubiquitin-proteasome pathway and proteasome inhibitors. Med Res Rev 21(4):245–273
Orrenius S, Gogvadze V, Zhivotovsky B (2007) Mitochondrial oxidative stress: Implications for cell death. Ann Rev Pharmacol Toxicol 47:143–183
Pedraza-Chaverri J, Cardenas-Rodriguez N, Orozco-Ibarra M, Perez-Rojas JM (2008) Medicinal aproperties of mangosteen (Garcinia mangostana). Food Chem Toxicol 46:3227–3239
Persson B, Kallberg Y (2013) Classification and nomenclature of the superfamily of short-chain dehydrogenases/reductases (SDRs). Chem Biol Interact 202(1–3):111–115
Ramaiya A, Li G, Petiwala SM, Johnson JJ (2012) Single dose oral pharmacokinetic profile of α-mangostin in mice. Curr Drug Target 13(14):1698–1704
Rieger AM, Nelson KL, Konowalchuk JD, Barreda DR (2011) Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J vis Exp 24(50):e2597
Sangkanu S, Mitsuwan W, Mahabusarakam W, Jimoh TO, Wilairatana P, Girol AP, Verma AK, de Lourdes Pereira M, Rahmatullah M, Wiart C, Siyadatpanah A (2021) Anti-Acanthamoeba synergistic effect of chlorhexidine and Garcinia mangostana extract or α-mangostin against Acanthamoeba triangularis trophozoite and cyst forms. Sci Rep 11(1):1–1
Sato A, Fujiwara H, Oku H, Ishiguro K, Ohizumi Y (2004) α-Mangostin induces Ca2+-ATPase-dependent apoptosis via mitochondrial pathway in PC12 cells. J Pharmacol Sci 95(1):33–40
Shimura H, Hattori N, Kubo SI, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T (2000) Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet 25(3):302–305
Siddiqui R, Rajendran K, Abdella B, Ayub Q, Lim SY, Khan NA (2020) Naegleria fowleri: differential genetic expression following treatment with Hesperidin conjugated with silver nanoparticles using RNA-Seq. Parasitol Res 119:2351–2358
Sifaoui I, Rodríguez-Expósito RL, Reyes-Batlle M, Rizo-Liendo A, Piñero JE, Bazzocchi IL, Lorenzo-Morales J, Jiménez IA (2019) Ursolic acid derivatives as potential agents against Acanthamoeba spp. Pathogen 8(3):130
Sifaoui I, Lopez-Arencibia A, Martín-Navarro CM, Reyes-Batlle M, Wagner C, Chiboub O, Mejri M, Valladares B, Abderrabba M, Pinero JE, Lorenzo-Morales J (2017) Programmed cell death in Acanthamoeba castellanii Neff induced by several molecules present in olive leaf extracts. PLoS One 12(8):e0183795
Simon SE, Lim HS, Jayakumar FA, Tan EW, Tan KO (2022) Alpha-Mangostin activates MOAP-1 tumor suppressor and mitochondrial signaling in MCF-7 human breast cancer cells. Evid Based Compl Altern Med 2022:1–2
Tadtong S, Viriyaroj A, Vorarat S, Nimkulrat S, Suksamrarn S (2009) Antityrosinase and antibacterial activities of mangosteen pericarp extract. J Health Res 23(2):99–102
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, Van Baren MJ, Salzberg SL, Wold BJ, Pachter L (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28(5):511–515
Trocha LK, Stobienia O (2007) Response of Acanthamoeba castellanii mitochondria to oxidative stress. Acta Biochim Pol 54(4):797–803
Villa E, Ricci JE (2016) How does metabolism affect cell death in cancer? FEBS Lett 283(14):2653–2660
Villa E, Proïcs E, Rubio-Patiño C, Obba S, Zunino B, Bossowski JP, Rozier RM, Chiche J, Mondragón L, Riley JS, Marchetti S (2017) Parkin-independent mitophagy controls chemotherapeutic response in cancer cells. Cell Rep 20(12):2846–2859
Wang C, Zhu J, Liu M, Yang Q, Wu J, Li Z (2018) De novo sequencing and transcriptome assembly of Arisaema heterophyllum Blume and identification of genes involved in isoflavonoid biosynthesis. Sci Rep 8(1):1–2
Wei H, Liu L, Chen Q (2015) Selective removal of mitochondria via mitophagy: distinct pathways for different mitochondrial stresses. Biochim Biophys Acta Mol Cell Res 1853(10):2784–2790
Wingett SW, Andrews S (2018) FastQ Screen: A tool for multi-genome mapping and quality control. F1000Research 7:1338
Wu Z, Lu Z, Ou J, Su X, Liu J (2020) Inflammatory response and oxidative stress attenuated by sulfiredoxin 1 in neuron like cells depends on nuclear factor erythroid 2 related factor 2. Mol Med Rep 22(6):4734–4742
Yang J, Yin Y (2020) PTEN in chromatin remodeling. Cold Spring Harbor Perspect Med 10(2):a036160
Young MD, Wakefield MJ, Smyth GK, Oshlack A (2010) Gene ontology analysis for RNA-seq: accounting for selection bias. Genom Biol 11(2):1–2
Zheng W, Rasmussen U, Zheng S, Bao X, Chen B, Gao Y, Guan X, Larsson J, Bergman B (2013) Multiple modes of cell death discovered in a prokaryotic (cyanobacterial) endosymbiont. PLoS ONE 8(6):e66147
Zhang KJ, Gu QL, Yang K, Ming XJ, Wang JX (2017) Anticarcinogenic effects of α-mangostin: a review. Plant Med 83(03/04):188–202
Acknowledgements
This research work was supported by Sunway University, Malaysia, and University of Karachi, Pakistan.
Funding
This research work was supported by Sunway University, Malaysia (Individual research grant, GRTIN-IRG-10–2022). Ruqaiyyah Siddiqui and Naveed Ahmed Khan are supported by the Air Force Office of Scientific Research (AFOSR), USA.
Author information
Authors and Affiliations
Contributions
SKO, AA and NAK conceived the study. AA, RS and KMK designed experiments. UA carried out all experiments under the supervision of AA, KOT, KMK, NAK, SKO, BSA, and RS. UA and AA analyzed data and prepared the first draft of the manuscript. All authors read, corrected and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Alpha-Mangostin is a xanthone with biological activities.
• α-Mangostin (AMS) inhibited viability of A. castellanii.
• AMS caused apoptosis through the mitochondrial pathway.
• AMS inhibited A. castellanii-mediated human cells cytopathogenicity.
• Transcriptome analysis showed significant expression of 826 genes.
• KEGG analysis showed autophagy, RAP1, AGE-RAGE and oxytocin signalling.
• PTEN,H3,ARIH1,SDR16C5,PFN,glnA GLUL, and SRX1) were found significant.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, U., Ong, SK., Tan, K.O. et al. Alpha-Mangostin and its nano-conjugates induced programmed cell death in Acanthamoeba castellanii belonging to the T4 genotype. Int Microbiol (2023). https://doi.org/10.1007/s10123-023-00450-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10123-023-00450-1