Abstract
Objective
The aim of this study was to clarify the risk of loss of independence (LOI) following gastrectomy in elderly patients with gastric cancer (GC).
Methods
In this prospective study, frailty was assessed preoperatively by a frailty index (FI) in 243 patients aged ≥ 65 years who underwent gastrectomy for GC between August 2016 and December 2020. Patients were assigned into two groups (high FI vs. low FI) to investigate frailty and the risk of LOI after gastrectomy for GC.
Results
Overall and minor (Clavien–Dindo classification [CD] 1, 2) complication rates were significantly higher in the high FI group, but the two groups had similar rates of major (CD ≥ 3) complications. The frequency of pneumonia was significantly higher in the high FI group. In univariate and multivariate analyses for LOI after surgery, high FI, older age (≥ 75 years), and major (CD ≥ 3) complications were independent risk factors. A risk score assigning 1 point for each of these variables was useful in predicting postoperative LOI (LOI: score 0, 7.4%; score 1, 18.2%; score 2, 43.9%; score 3, 100%; area under the curve [AUC] = 0.765.)
Conclusions
LOI after gastrectomy was independently associated with high FI, older age (≥ 75 years), and major (CD ≥ 3) complications. A simple risk score assigning points for these factors was an accurate predictor of postoperative LOI. We propose that frailty screening should be applied for all elderly GC patients before surgery.
Similar content being viewed by others
Introduction
As the population ages, elderly individuals with cancer diagnoses are not a minority but a majority, and surgeons are increasingly concerned with improving outcomes in elderly cancer patients. Curative gastrectomy with lymph node dissection is the main treatment for patients with gastric cancer (GC) [1]. In Japan, the procedure for gastrectomy is standardized and safe, with low mortality [2]. However, gastrectomy can be problematic in older patients because of poor nutrition status or muscle weakness after surgery, and older age itself has been associated with worse outcomes after gastrectomy for GC [3,4,5].
Surgery poses greater risk in the elderly because they may have more comorbidities, decreased stress tolerance, decreased physical function, and decreased cognitive function [6]. However, the degree of risk varies widely among individual patients. While some elderly are so vulnerable that surgeons are hesitant to perform surgery, others are robust and highly active, and “calendar age” is not an accurate determinant in assessing operative risk. In addition, for elderly patients with declining physical function, the ability to lead an independent life after surgery is an important issue. Since loss of independence (LOI) after surgery is matter of life or death for the elderly, preoperative assessment of the risk of postoperative LOI should be a priority, and postoperative care should be planned accordingly.
Frailty has recently received much attention as a geriatric assessment for the elderly. Frailty is a multifactorial challenge characterized in part as a biological syndrome of decreased reserve and resistance to stressors that can lead to increased risk of poor health outcomes including worsening mobility or loss of other activities of daily living (ADLs), hospitalization, and death [7, 8]. How best to define frailty on its own terms is controversial [9,10,11], but one method uses a frailty index [12]. Rockwood et al. define frailty as an accumulation of physical and functional impairments (disease, psychological disorders, nutritional disorders, and physiologic abnormalities) that affect physical capacity, ADLs, and instrumental activities of daily living (IADLs) [13]; they proposed that factors related to frailty could be assessed cumulatively to calculate a frailty index (FI) [8, 14]. So far, there are no published reports studying the impact of preoperative frailty, as assessed by FI, on the risk of postoperative LOI in elderly GC patients undergoing gastrectomy.
In this study, we compared preoperative FI and short-term outcomes in GC patients undergoing gastrectomy. We investigated factors that contributed to the risk of LOI for elderly GC patients after surgery, and based on these findings, we aimed to develop a simple scoring system for preoperative assessment of the risk of postoperative LOI.
Methods
Patients and data collection
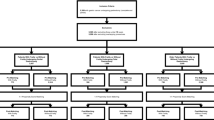
In this prospective study, we preoperatively conducted frailty assessments for a total of 243 GC patients aged 65 years or older who agreed to participate in the study and were scheduled for gastrectomy for GC between August 2016 and December 2020. Informed consent for participation in this study was obtained from all included patients, and all patients completed the frailty assessment on the day before surgery. Among these patients, 8 were already certified for nursing care under long-term care insurance (LTCI) because of disability; 7 had only laparotomy or underwent surgery such as bypass with no resection; 7 had cancer at the esophago-gastric junction (EGJ); 6 had double primary cancers; 5 had gastrectomy after chemotherapy for stage 4 disease; 2 died from postoperative complications; and 1 had local resection of GC due to uncontrolled bleeding from the tumor. Therefore, 207 patients undergoing gastrectomy for GC were finally included in the analysis (Fig. 1). We extracted the characteristics of each patient from original medical records, operative reports, and nursing charts. Patient data including age, gender, BMI, tumor stage, procedure type (distal, proximal, total), approach (open or laparoscopic/robot assisted), operative time, blood loss, intraoperative blood transfusion, lymphadenectomy, complications, reoperation, postoperative hospital days, and enrollment in LTCI after surgery were evaluated for the study. Patients with pStage 1 tumors were scheduled for outpatient follow-up at least once every 6 months, and those with pStage 2 or higher were to follow up every 2 to 3 months. Whether or not patients were receiving long-term care services under the LTCI system was confirmed at the outpatient clinic or by telephone. The TNM stage was described based on the Japanese classification of gastric carcinoma (15th edition) [15]. The study was approved by the Institutional Review Board of the Osaka City General hospital (IRB approval number: 1606029).
Frailty assessment
In this study, we adopted a cumulative deficit FI method proposed by Rockwood et al. to assess frailty [8]. The principle of this method is that health deficits, i.e., symptoms, disease, comorbidities, acquired disabilities, laboratory abnormalities, and others, are additive in their contribution to frailty. Referring to reports by Joseph et al. we used a cumulative FI comprising 50 variables related to age, comorbidity and medical history, social activity, ADLs, IADLs, nutritional status, physical function, and general mood (Table 1) [16]. Each variable had a value of 0 to 1, and the cumulative points were divided by 50 to obtain the FI. For example, if cumulative frailty points = 8, the frailty index = 8 ÷ 50 = 0.16. Thus, the higher the FI, the more advanced the frailty. Frailty assessments were completed the day before surgery because, per our protocol, patients are admitted to the hospital the day before their planned surgery. The patients were given questionnaires on the ward upon admission and those who consented to this study were asked to complete the questionnaire on the same day. The medical staff then measured height and weight of patients. Left and right grip strength were also measured using a digital grip strength meter, and the higher value was adopted. Completed questionnaires were collected by the staff later that day. Routine blood tests were also performed on the day of admission.
Receiver operating characteristic (ROC) curves were generated for multiple logistic regression analysis using LOI (initiation of LTCI services) as the endpoint; an optimal cutpoint for FI was then determined, and patients were assigned to either the high FI or low FI group for further analysis.
Long-term care insurance system
The LTCI system in Japan is a public program that provides nursing care services for citizens who need assistance in their daily lives because of declining physical or cognitive function [17, 18]. The LTCI program is administered by local governments, and eligibility is determined by computerized assessment analyzed by trained medical and governmental officials. Elderly citizens who request and are found to be in need of long-term care receive nursing care certification (not cash) for services matching their disability. Depending on the level of care needed, services may be provided at home or in an extended care facility [19]. All citizens over the age of 40 years are required to contribute to the public LTCI, which funds most of the costs of the service on behalf of the person being certified. Therefore, people who are certified can use the service regardless of economic status. In this study, LTCI system users were determined to have decreased physical independence.
Selection criteria for open and laparoscopic/robot-assisted surgery
From April 2016 to March 2019, we selected open surgery for patients who had scirrhous GC or bulky nodal metastasis, possibility of T4b, duodenal invasion, or esophageal invasion, and for the patients who required total gastrectomy with splenectomy. Otherwise, laparoscopic/robot-assisted surgery was selected. Since April 2019, we consider robot-assisted surgery for all cases except for scirrhous GC. Robot-assisted distal gastrectomy (RDG) was adopted for cT1N0M0 from January 2017 and robot-assisted total gastrectomy (RTG) from April 2018.
Definitions
The severity of complications was graded according to the Clavien–Dindo (CD) classification [20], with CD grade 1 or 2 complications considered minor and CD grade 3 or higher complications considered major. LOI was defined based on the requirement for healthcare support after surgery; namely, whether or not patients newly became LTCI system users.
Statistical analysis
Descriptive variables in the high FI and low FI groups at baseline were expressed as means and standard deviations or medians and ranges for continuous variables and numerical values and percentages for categorial variables. These variables were compared via Mann–Whitney U test and chi-squared test. Receiver operating characteristic curves that correlated the true- and false-positive rates (sensitivity and [1–specificity]) were generated to obtain the optimal cutpoint for FI to predict LOI after surgery. To identify the risk factors for LOI, the following covariates were included in the univariate analysis: age, gender, BMI, extent of gastrectomy, nature of procedure (open or laparoscopic/robot-assisted), pathologic staging (pStage), major complications, and frailty. Of those, only variables showing statistical significance (p < 0.05) were entered into multivariate analysis. The Cochran–Armitage trend test was applied to categorical variables to evaluate trends in the incidence of LOI by score according to the number of applicable risk factors. All reported p-values were two-sided, and p < 0.05 represented statistical significance. The statistical computations relied on standard software (JMP v10; SAS Institute Inc., Cary, NC, USA).
Results
Distribution of frailty index values
The FI distribution is shown in Fig. 2. The higher the FI, the more advanced the frailty. The FI values ranged from 0.020 to 0.465 (mean 0.175, median 0.160, first quartile 0.110, third quartile 0.225). The area under the ROC curve (AUC) in multiple logistic regression analysis with LOI as the endpoint was 0.7554. The projected LOI was optimal at an FI value of 0.194 (sensitivity 0.6981; specificity 0.7013). Hence, this value was adopted as the cutpoint for stratifying the patients by low FI (≤ 0.194) or high FI (> 0.194). The rate of LOI after surgery was significantly higher in the high FI group vs. the low FI group (44.6 vs. 12.9%, p < 0.001).
Patient characteristics
The clinical profiles of the two groups are shown in Table 2. As compared with the low FI group, the high FI group had more elderly patients, less frequent minimally invasive surgery, longer operation time, more frequent morbidities and reoperation, and longer hospital stay.
The time from discharge to LTCI certification was significantly shorter in the high FI group compared to the low FI group (p = 0.034).
Univariate and multivariate analyses for predictors of loss of independence after gastrectomy
Results of univariate and multivariate analyses for LOI risk are shown in Table 3. On univariate analysis of 8 clinicopathologic factors, high FI, age ≥ 75, open surgical approach, and CD ≥ 3 proved significantly predictive of LOI. In the multivariate analysis, high FI (odds ratio [OR], 4.23; 95% confidence interval [CI], 2.05–9.08; p < 0.001), age ≥ 75, and CD ≥ 3 were independent risk factors for LOI.
Predictive score for loss of independence
A 3-point scoring system for predicting LOI was tested, with high FI, age ≥ 75, and CD ≥ 3 each contributing 1 point. The relationship between this predictive score and LOI after surgery is shown in Fig. 3. The proportion of patients with LOI was 7.4% in those with 0 points, 18.2% in those with 1 point, 43.9% in those with 2 points, and 100% in those with 3 points. We found that an increase in the number of applicable risk factors was associated with a corresponding rise in the incidence of postoperative LOI (Cochran-Armitage trend test, p < 0.001). Additional analysis of this scoring system demonstrated good discrimination for predicting LOI in this context, with AUC = 0.765 (Fig. 4).
Discussion
In this analysis, high FI was an independent predictor of postoperative LOI in elderly GC patients undergoing gastrectomy, and the risk factors for LOI after gastrectomy were high FI, older age (≥ 75 years), and CD ≥ 3. A simple 3-point scoring system using these factors was a useful tool for predicting postoperative LOI.
The physical independence of the vulnerable elderly after surgery is of particular interest to surgeons because surgery in the elderly is often unduly invasive and irreversibly drains the patient’s strength. Geriatric patients may be less active after discharge from the hospital and closer to being bedridden, and they may not have the expected outcomes even after a curative surgery. In these patients, besides achieving cure, maintaining preoperative activity levels is paramount. Therefore, we wanted to investigate the utility of a frailty assessment for predicting postoperative LOI in elderly GC patients. Our results suggest that even if GC is cured, patients with high FI, age ≥ 75 years, and CD ≥ 3 may have disability and LOI after gastrectomy. Preoperative evaluations are mandatory for selecting appropriate treatment. When we did additional univariate and multivariate analyses excluding major complications (≥ CD3), high FI was still independently associated with LOI (supplementary Table 1). This emphasizes the need for careful decision-making for surgery and informed communication between doctors and patients before surgery. Our simple 3-point scoring system can provide an additional risk assessment for LOI. Although the application of nomograms to risk assessment could be made even more effective by weighting each risk factor, in this study, we used only the sum of the number of applicable risk factors with the aim of testing the usefulness of a simplified risk assessment method that could be accessible to all surgeons. This simplified risk assessment method reached a high degree of accuracy. We believe this preoperative frailty screening has a value to be validated in the multi-center study.
There are several reports on frailty assessment in the elderly [7, 8, 21]. Nowadays, there are two main frameworks: the physical phenotype proposed by Fried et al. and the multidomain phenotype proposed by Rockwood et al. [7, 13]. The multidomain phenotype is an index of accumulated health deficits including impaired function, comorbidities, and mood and laboratory abnormalities, and is based on a principle that the more variables assessed, the more accurate the frailty estimate becomes. Searle et al. have reported that a cumulative multidomain FI can accurately assess frailty by evaluating at least 30 frailty-related variables [8]. We adopted a multidomain phenotype FI based on 50 frailty-related variables and encompassing medical history, ADLs, IADLs, and social and psychological factors, which has been reported and found to be a comprehensive and highly reliable assessment of frailty [16]. Although there is still debate over which method is ideal, we found that the FI we chose was an effective tool for predicting postoperative LOI in elderly GC patients.
Knowing that major complications (CD ≥ 3) are a risk factor for LOI suggests that an uneventful postoperative course would be favorable for avoiding postoperative LOI, and it is important for the surgeon to not only pursue a cure, but also an approach and a meticulous procedure that reduces the risk of complications. With the advance of laparoscopic techniques and equipment, laparoscopic distal gastrectomy (LDG) has been found a safe and feasible alternative to open distal gastrectomy (ODG) [22,23,24]. LDG is now an option as a standard procedure for clinical Stage 1 GC according to the Japanese Treatment Guidelines 2018 (5th edition) [1]. Performed with skill, LDG can have the advantage of a shorter hospital stay compared to ODG, which could favor an early return to regular daily life [25]. On the other hand, laparoscopic total and proximal gastrectomy are technically difficult and real-world data are showing high complication rates [26, 27]. In this study, many patients with early GC underwent laparoscopic/robot-assisted surgery, and it is unclear whether laparoscopic/robot-assisted surgery was useful for avoiding LOI. More research will be needed to examine this issue.
Postoperative pneumonia was more common in the high FI patients in our study. Reported risk factors for postoperative pneumonia after surgery for GC include advanced age, poor nutritional status, total gastrectomy, time to first meal after surgery, extended operation time, D2 lymph node dissection, advanced tumor stage, and predicted vital capacity (VC) [28,29,30,31]. It is difficult to prevent pneumonia via the surgical technique because the extent of gastrectomy and lymph node dissection is essentially predetermined by the location and the progression of the tumor. A choice for limited surgery could follow consideration of LOI risk and possibility of GC mortality, and in recent years, enhanced recovery after surgery (ERAS) has been recommended for improved perioperative outcomes, with intent to minimize postoperative pain, promote recovery, reduce complications, and shorten hospital stays [32]. Preoperative intervention to relieve frailty is presently of limited value. Some authors have reported that nutrition therapy and resistance exercise for community-dwelling elderly are effective for improving physical function, blood components, and body composition [33, 34]. However, the clinical benefit of perioperative nutritional intervention and exercise in frail patients with GC is unclear.
We recognize some limitations in our study. First, this study was performed at a single facility in Japan, and the number of enrolled patients was not large. Additional larger-number, multi-institutional studies will be needed to validate and standardize the proposed scoring system. Second, the application for LTCI services may seem subjective, as it is initiated by the patient based on the patient’s complaint. However, a documented doctor’s opinion is required to apply for LTCI services, and LTCI services are not recommended for patients living independently who do not require services. In addition, a trained medical officer, who makes the determination on whether care is needed, must visit and interview the patient. Thus, the LTCI certification system is designed to maintain objectivity. Third, the defined LOI, that is, becoming certified for LTCI services after surgery, may not be permanent. Forth, the LTCI nursing care certification is a Japanese public health initiative, and the evaluation for eligible LOI in the elderly here may not be applicable in other Asian countries or in Western countries. However, although insurance systems may vary by country, the implementation of health care programs such as LTCI for the elderly will be the responsibility of every government as the world population ages more and more in the future. Finally, a deficit accumulation method was used for the FI in this study, and the relationship between frailty and outcomes should be confirmed using other frailty assessment tools.
In conclusion, higher FI in association with more severe complications and advanced age increase the risk of LOI after surgery in elderly GC patients. A simple scoring method, based on high FI, older age (≥ 75 years), and complications (CD ≥ 3) was an accurate predictor of LOI in elderly GC patients undergoing gastrectomy. As a high FI in elderly patients with GC can adversely affect their postoperative independence, we propose that preoperative frailty screening should be conducted in all elderly GC patients undergoing gastrectomy.
References
Japanese gastric cancer treatment guidelines. 5th edition. Gastric Cancer. 2018;2021(24):1–21.
Kakeji Y, Takahashi A, Hasegawa H, Ueno H, Eguchi S, Endo I, et al. Surgical outcomes in gastroenterological surgery in Japan: report of the national clinical database 2011–2018. Ann Gastroenterol Surg. 2020;4:250–74.
Sakurai K, Kubo N, Tamamori Y, Tamura T, Toyokawa T, Tanaka H, et al. Long-term survival estimates in older patients with pathological stage I gastric cancer undergoing gastrectomy: duocentric analysis of simplified scoring system. J Geriatr Oncol. 2019;10:604–9.
Wakahara T, Ueno N, Maeda T, Kanemitsu K, Yoshikawa T, Tsuchida S, et al. Impact of gastric cancer surgery in elderly patients. Oncology. 2018;94:79–84.
Yu J, Hu J, Huang C, Ying M, Peng X, Wei H, et al. The impact of age and comorbidity on postoperative complications in patients with advanced gastric cancer after laparoscopic D2 gastrectomy: results from the Chinese laparoscopic gastrointestinal surgery study (CLASS) group. Eur J Surg Oncol. 2013;39:1144–9.
Sakurai K, Muguruma K, Nagahara H, Kimura K, Toyokawa T, Amano R, et al. The outcome of surgical treatment for elderly patients with gastric carcinoma. J Surg Oncol. 2015;111:848–54.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56.
Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24.
Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, et al. Frailty: an emerging research and clinical paradigm–issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–7.
Fisher AL. Just what defines frailty? J Am Geriatr Soc. 2005;53:2229–30.
Whitson HE, Purser JL, Cohen HJ. Frailty thy name is Phrailty? J Gerontol A Biol Sci Med Sci. 2007;62:728–30.
Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–36.
Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–7.
Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26.
Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1–21.
Joseph B, Pandit V, Zangbar B, Kulvatunyou N, Hashmi A, Green DJ, et al. Superiority of frailty over age in predicting outcomes among geriatric trauma patients: a prospective analysis. JAMA Surg. 2014;149:766–72.
Tamiya N, Noguchi H, Nishi A, Reich MR, Ikegami N, Hashimoto H, et al. Population ageing and wellbeing: lessons from Japan’s long-term care insurance policy. Lancet. 2011;378:1183–92.
Tsutsui T, Muramatsu N. Care-needs certification in the long-term care insurance system of Japan. J Am Geriatr Soc. 2005;53:522–7.
Tanaka S, Iida H, Ueno M, Hirokawa F, Nomi T, Nakai T, et al. Preoperative risk assessment for loss of independence following hepatic resection in elderly patients: a prospective multicenter study. Ann Surg. 2021;274:e253–61.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
Satake S, Senda K, Hong YJ, Miura H, Endo H, Sakurai T, et al. Validity of the Kihon Checklist for assessing frailty status. Geriatr Gerontol Int. 2016;16:709–15.
Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, et al. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan clinical oncology group study JCOG0912. Gastric Cancer. 2017;20:699–708.
Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage i gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5:506–13.
Katai H, Mizusawa J, Katayama H, Morita S, Yamada T, Bando E, et al. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5:142–51.
Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–7.
Kodera Y, Yoshida K, Kumamaru H, Kakeji Y, Hiki N, Etoh T, et al. Introducing laparoscopic total gastrectomy for gastric cancer in general practice: a retrospective cohort study based on a nationwide registry database in Japan. Gastric Cancer. 2019;22:202–13.
Etoh T, Inomata M, Shiraishi N, Kitano S. Minimally invasive approaches for gastric cancer-Japanese experiences. J Surg Oncol. 2013;107:282–8.
Kiuchi J, Komatsu S, Ichikawa D, Kosuga T, Okamoto K, Konishi H, et al. Putative risk factors for postoperative pneumonia which affects poor prognosis in patients with gastric cancer. Int J Clin Oncol. 2016;21:920–6.
Kimura R, Moriyama T, Ohuchida K, Shindo K, Nagai S, Ohtsuka T, et al. Risk factors for postoperative pneumonia after laparoscopic gastrectomy in patients aged 75 years and over with gastric cancer. Asian J Endosc Surg. 2021;14:408–16.
Ntutumu R, Liu H, Zhen L, Hu YF, Mou TY, Lin T, et al. Risk factors for pulmonary complications following laparoscopic gastrectomy: A single-center study. Medicine (Baltimore). 2016;95: e4567.
Inokuchi M, Kojima K, Kato K, Sugita H, Sugihara K. Risk factors for post-operative pulmonary complications after gastrectomy for gastric cancer. Surg Infect (Larchmt). 2014;15:314–21.
Wee IJY, Syn NL, Shabbir A, Kim G, So JBY. Enhanced recovery versus conventional care in gastric cancer surgery: a meta-analysis of randomized and non-randomized controlled trials. Gastric Cancer. 2019;22:423–34.
Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, et al. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc. 2012;60:16–23.
Kim H, Kim M, Kojima N, Fujino K, Hosoi E, Kobayashi H, et al. Exercise and nutritional supplementation on community-dwelling elderly Japanese women with sarcopenic obesity: a randomized controlled Trial. J Am Med Dir Assoc. 2016;17:1011–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors received no specific funding for this work and have declared that no competing interest exists.
Informed consent
All the procedures followed were in accordance with the ethical standards of the responsible Committees on human experimentation (Institutional and National) and with the Helsinki Declaration of 1964 and later versions. Informed consent for inclusion in the study was obtained from all the patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sakurai, K., Kubo, N., Hasegawa, T. et al. Risk factors of “loss of independence” in elderly patients who received gastrectomy for gastric cancer. Gastric Cancer 26, 638–647 (2023). https://doi.org/10.1007/s10120-023-01376-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-023-01376-3