Abstract
Background
Gastric cancer (GC) is a leading cause of cancer-related mortality worldwide, because of the low efficacy of current therapeutic strategies. Estrogen-related receptor γ (ERRγ) was previously showed as a suppressor of GC. However, the mechanism and effective therapeutic method based on ERRγ is yet to be developed.
Methods
The expression levels of ERRγ, EZH2, and FOXM1 were detected by immunohistochemistry, qRT-PCR, and western blot. The regulatory mechanisms of ERRγ and FOXM1 were analyzed by ChIP, EMSA, and siRNA. The effects of EZH2 inhibitor (GSK126) or/and ERRγ agonist (DY131) on the tumorigenesis of gastric cancer cell lines were examined by cell proliferation, transwell migration, wound healing, and colony formation assays. Meanwhile, the inhibitory effects of GSK126 or/and DY131 on tumor growth were analyzed by xenograft tumor growth assay.
Results
The expression of ERRγ was suppressed in tumor tissues of GC patients and positively correlated with prognosis, as opposed to that of EZH2 and FOXM1. EZH2 transcriptionally suppressed ERRγ via H3K27me3, which subsequently activated the expression of master oncogene FOXM1. The combination of GSK126 and DY131 synergistically activated ERRγ expression, which subsequently inhibited the expression of FOXM1 and its regulated pathways. Synergistic combination of GSK126 and DY131 significantly inhibited the tumorigenesis of GC cell lines and suppressed the growth of GC xenograft.
Conclusion
The FOXM1 signaling pathway underlying the ERRγ-mediated gastric cancer suppression was identified. Furthermore, combined treatment with EZH2 inhibitor and ERRγ agonist synergistically suppressed GC progression by inhibiting this signaling pathway, suggesting its high potential in treating GC patients.
Similar content being viewed by others
Introduction
Gastric cancer (GC), also known as stomach cancer, is a disease in which malignant cells form in the lining of the stomach, and is anatomically divided into non-cardia and cardia gastric cancers [1]. GC has a high incidence (> 20 per 100,000 in men) in China, Japan, Latin America, and Eastern Europe [2]. According to 2012 statistics, 260,000 and 691,000 cases corresponded to cardia and non-cardia gastric cancers, respectively [3]. According to 2018 estimates, GC caused over 780,000 deaths and ranked as the third leading cause of cancer death worldwide [4]. In most cases, GC is diagnosed at an advanced stage due to its asymptomatic features at its early stages, which results in poor prognosis of patients after resection [5]. Therefore, identification of underlying mechanisms and key genes during the onset of GC is important for developing innovative strategies for its prevention, early detection, and treatment.
GC is a multifactorial cancer, and both genetic and environmental factors have a role in its etiology. Common risk factors for both cardia and non-cardia GC include, older age, male sex, obesity, family history, tobacco smoking, radiation, and Helicobacter pylori (H. pylori) infection [1]. In particular, H. pylori infection is the most positively associated with GC onset, causing 65–80% of all GC cases annually [6]. The development of GC is a consequence of complex interactions of microbial agents with environmental and host factors, resulting in the dysregulation of multiple oncogenic and tumor-suppressing signaling pathways [7, 8]. Horvat et al. showed that H. pylori induced ubiquitination and degradation of the tumor suppressor p14ARF, inhibiting autophagy in GC [9]; meanwhile, Hu et al. observed that H. pylori down-regulated tumor suppressor activities of RACK1 [10]. Additionally, Holokai et al. demonstrated that H. pylori induced PD-L1 expression in gastric epithelial cells, which protected cells from the immune response, and promoted the progress of premalignant lesions to gastric cancer [11]. Additionally, bacteria such as Peptostreptococcus stomatis, Streptococcus anginosus, Parvimonas micra, Slackia exigua, Dialister pneumosintes, Clostridium, and Fusobacterium species are also frequently abundant in patients with GC [12, 13]. Besides bacterial infection, many mutations in genes, e.g., CDH1, CTNNA1, TP53, APC, or STK11, have also been identified as causes of GC [14,15,16]. Therefore, the molecular characteristics of GCs are complex and heterogeneous. Recent comprehensive molecular profiling of GC proposed four molecular subtypes in GC patients: Epstein-Barr virus-associated, microsatellite instable, chromosomal instable, and genomically stable carcinomas [17, 18]. The biological complexity of GC has hampered the discovery of universal molecular targets and subsequent implementation of effective targeted therapies. Thus, a better understanding of the molecular drivers of GC pathophysiology is essential for the identification of novel and effective therapeutic targets. A recent study indicated that ERRγ was generally expressed in low levels in GC samples, and the ERRγ agonist, DY131, significantly inhibited proliferation of GC cells [19]. However, the regulatory mechanism of ERRγ in GC is still unclear. In general, epigenetic alterations, including DNA methylation and histone modification (e.g., H3K27me3), are deeply involved in the development and progression of gastric cancers [20]. Thus, we mainly focused on the regulatory mechanism of ERRγ in GC on the aspect of epigenetic changes.
Currently, some identified molecules have been used as therapeutic targets in GC, e.g., epidermal growth factor receptor (EGFR) and the EGFR-related receptor-HER2, c-MET, mTOR, Pan-HER, VEGF, and PD-1 [5]. However, most of the treatments targeting these molecules were unsatisfactory. The EGFR protein is overexpressed in most GCs; however, the trials that used anti-EGFR antibodies such as cetuximab (EXPAND Trial), and panitumumab (REAL3 Trial) failed to improve survival of patients [21, 22]. Trastuzumab, a monoclonal antibody interfering with the activation of HER2, was the first targeted agent that significantly improved patient survival in the treatment of GC; however, its major drawback was the emergence of trastuzumab resistance [23]. Bevacizumab, a monoclonal antibody against VEGF-A, failed in phase 3 trial [24]. Clinically, combination therapy is superior to monotherapy in cancer treatment, as discussed in the following sentences [5, 25]. The combination of MAOA inhibitors and the survivin suppressants (YM155 and SC144) showed significant synergism in the inhibition of prostate cancer [26]. The combination of simvastatin and metformin synergistically inhibited endometrial cancer cell growth [27]. The combination of trastuzumab with cisplatin resulted in promising antitumor activity and manageable toxic effects in patients with HER2 overexpressing advanced GC [28]. The combination of two antibodies (pertuzumab and trastuzumab) significantly enhanced therapy efficacy in HER2 positive GC [29]. Although few combined therapeutic strategies for GC treatment have been developed, more effective target molecules and treatment strategies are still needed, because of the complex and heterogeneous molecular characteristics of GC.
In this study, we investigated the mechanism of the tumor suppressor ERRγ and demonstrated the synergistic inhibitory effects of two chemicals targeting the identified pathway.
Materials and methods
Xenograft tumor growth assay
Female nude mice were purchased from HUNAN SJA LABORATORY ANIMAL company (Hunan, China) and maintained according to the animal experimentation guidelines and following the Regulations for the administration of Affairs Concerning Experimental Animals of Guangdong Province, China. All mouse experiments were approved and supervised by the Institutional Animal Care and Use Committee of SUN YAT-SEN University (IACUC No. L102022018120E). The mice (6–8 weeks old) were subcutaneously injected with MGC803 cells to establish GC tumors. When tumors reached a volume of 100 mm3, mice were randomly divided into four groups (5 mice/group) and subcutaneously injected with or without 30 mg/kg of DY131 or/and 50 mg/kg of GSK126 for 20 days. At the time of sacrifice, tumor volume and weight, and body weight were recorded.
Statistical analysis
All statistics were performed using SPSS 16.0 (IBM, USA). All variables were plotted using GraphPad Prism 7.0 (USA). Statistically significant differences among more than two groups, which corresponded to values of ***p < 0.001, **p < 0.01, or *p < 0.05, were determined using one-way analysis of variance, followed by Bonferroni’s multiple comparison tests. Overall survival of patients was determined using the Kaplan–Meier method. The genomic data of GC patients are available from the TCGA database and NCBI Gene Expression Omnibus (GEO) under accession numbers (GSE80239, GSE85431, GSE14208 and GSE26253).
Results
ERRγ expression is transcriptionally suppressed by EZH2 via H3K27me3
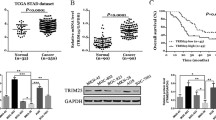
Previous reports showed that low expression of ERRγ in several types of cancers predicts poor prognosis [19, 29, 30]. In this study, it was further validated in 375 Chinese GC patients (Fig. S-1). Furthermore, overexpression of ERRγ significantly inhibited cell proliferation and migration, wound healing, and colony formation in GC cell lines, AGC and MGC803 (Fig. S2), suggesting that ERRγ was an important regulator of GC onset. Thus, it is important to clarify the regulatory mechanisms of ERRγ.
The database mining of GEO showed that the treatment of LnCaP, DU145, and Kelly cells with GSK126 (EZH2 inhibitor) significantly up-regulated (p < 0.05) the expression of ESRRG (Fig. 1a). Meanwhile, a significant negative correlation between the levels of ERRγ and EZH2 in GC patients was observed in this study, suggesting that EZH2 might be a negative regulator of ERRγ expression in GC (Fig. 1b).
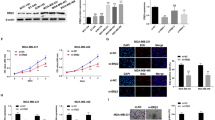
EZH2 negatively regulated the expression of ERRγ by H3K27me3. a Relative ESRRG mRNA levels in LNCaP and DU145 cells treated or not with GSK126 (GEO database: GSE80239 and GSE85431). b Correlation analysis of the expression of ERRγ and EZH2 in 78 gastric adenocarcinoma patients. c The ESRRG mRNA levels in EZH2 knockdown MGC803 and SGC7901 cells. d The protein levels of ERRγ in EZH2 knockdown MGC803 and SGC7901 cells. e The ESRRG mRNA levels in EZH2 overexpressed NCI-N87 and HGC27 cells. f The protein levels of ERRγ in EZH2 overexpressed NCI-N87 and HGC27 cells. g The protein levels of ERRγ and H3K27me3 in AGS and MGC803 cells after GSK126 treatment. h ChIP analysis of the enrichment of H3K27me3 on the ESRRG promoter after EZH2 overexpression or knockdown (MGC803). kb is the distance from the TSS site of ESRRG. All experiments were performed in triplicate, and the values given represent the mean ± SD of three independent experiments. Statistical significance was defined as *p < 0.05, **p < 0.01, ***p < 0.001
To investigate if EZH2 negatively regulates the expression of ERRγ in GC, the protein levels of ERRγ and EZH2 in several gastric cancer cells were firstly analyzed by Western blot (Fig. S3). Then, two of GC cell lines with high EZH2 expression (MGC803 and SGC7901) were used in EZH2 knockdown experiments and two of GC cell lines with low EZH2 expression (NCI-N87 and HGC27) were used in EZH2 overexpression experiments. Expectedly, knockdown of EZH2 significantly up-regulated the expression of ERRγ in MGC803 and SGC7901 cells (Fig. 1c, d), while overexpression of EZH2 significantly down-regulated the expression of ERRγ in NCI-N87 and HGC27 cells (Fig. 1e, f). Consistently, the EZH2 inhibitor (GSK126) significantly up-regulated the expression of ERRγ in AGS and MGC803 cells (Fig. 1g). Furthermore, ChIP analysis showed that H3K27me3, epigenetic modification to the DNA packaging protein histone H3, was enriched in the upstream region of ERRγ, when the EZH2 was overexpressed (Fig. 1h). Moreover, EZH2 knockdown significantly inhibited cell proliferation, wound healing, cell migration, and colony formation in AGC and MGC803 cell lines (Fig. S4). Finally, EZH2 was highly expressed in tumor tissues of 375 GC patients and its expression was negatively correlated with overall survival rate of the patients (Fig. S5). Altogether, these results suggested that the expression of ERRγ and thus prognosis of GC patients were negatively regulated by EZH2 expression.
ERRγ transcriptionally suppresses the expression of master oncogene FOXM1
To investigate the mechanism of ERRγ suppression in GC, oncogenes which may be directly regulated by ERRγ were investigated. Two ERRγ binding elements (ERRE) were found upstream of the master oncogene, FOXM1 (Fig. 2a), and further confirmed by ChIP and EMSA assays. ChIP analysis showed significant binding of ERRγ on two ERRE sites when ERRγ was overexpressed in MGC803 cells (Fig. 2b). Furthermore, EMSA analysis indicated that ERRγ directly bound to ERRE upstream of FOXM1 (Fig. 2c). On the other hand, when ERRγ proteins were overexpressed in MGC803, BGC823 and SGC7901 cells, the mRNA and protein levels of FOXM1 were significantly down-regulated (p < 0.01) (Fig. 2d, e).
ERRγ inhibited FOXM1 expression via binding to its upstream ERRE elements. a Location and sequences of two ERREs in the FOXM1 promoter. b The enrichment of ERRγ on the FOXM1 promoter was detected by ChIP assay after ERRγ overexpression. c EMSA detected the binding of ERRγ to the ERRE element on FOXM1 promoter. d, e The mRNA (d) and protein (e) levels of FOXM1 were detected by RT-qPCR and western blot after overexpression of ERRγ. All experiments were performed in triplicate, and the values given represent the mean ± SD of three independent experiments. Statistical significance was defined as **p < 0.01 or ***p < 0.001
Later, FOXM1 data were analyzed in TCGA and GEO databases. According to TCGA database, the expression of FOXM1 was significantly higher in tumors than in normal tissues (p < 0.001) (Fig. S6a). Consistently, GEO database (GSE14208 and GSE26253) indicated significant lower overall survival rate of GC patients with higher expression levels of FOXM1 (Fig. S6b). Altogether, ERRγ transcriptionally inhibited the expression of FOXM1, which may be critical for GC patient prognosis.
Inhibition of EZH2 and activation of ERRγ synergistically inhibits GC cell proliferation and migration
Because EZH2 and ERRγ were negatively and positively correlated with prognosis of GC patients, respectively, and EZH2 negatively regulated the expression of ERRγ, we investigated the effects of EZH2 inhibitor GSK126 and ERRγ agonist DY131, when administered separately or combined, on the tumorigenesis of GC cells. Addition of DY131 weakly inhibited cell proliferation and migration, lactate production, wound healing, and colony formation (Fig. S7), which might be caused by low expression of ERRγ in GC cells. Regarding administration of GSK126, cell proliferation and migration, lactate production, wound healing, and colony formation, were greatly inhibited but required concentrations up to 20 μΜ (Fig. S8). Surprisingly, the combined low doses of DY131 (5 μΜ) and GSK126 (10 μΜ) greatly and synergistically, inhibited cell migration and proliferation, lactate production wound healing, and colony formation (Fig. 3). After 72 h treatment with 5 μΜ DY131 or 10 μΜ GSK126, the viabilities of AGS, MGC803, and NCI-N87 cells were approximately 50, 80, and 80%, respectively (Fig. 3a). However, AGS, MGC803, and NCI-N87 cells were less than 10% viable under treatment with 5 μΜ DY131 plus 10 μΜ GSK126 (Fig. 3a). Consistently, similar synergistic effects of DY131 and GSK126 were observed in lactate production, cell migration, wound healing, and colony formation assay (Fig. 3b–e). Taken together, these data suggested that inhibition of EZH2 and activation of ERRγ synergistically inhibited the tumorigenesis of GC cells.
The effects of GSK126 and/or DY131 on the cell viability and migration, lactic acid production, wound healing, and colony formation of GC cell lines. a The cell viability of gastric cancer cell lines treated with DY131 or/and GSK126 were detected by CCK8 assay. b The production of lactic acid in gastric cancer cell lines treated with DY131 or/and GSK126 was detected by lactic acid assay kit. c–e Cell migration (c), wound healing (d) and colony formation (e) of gastric cancer cell lines treated with DY131 or/and GSK126. All experiments were performed in triplicate, and the values given represent the mean ± SD of three independent experiments. Statistical significance was defined as *p < 0.05, **p < 0.01, ***p < 0.001
Inhibition of EZH2 and activation of ERRγ synergistically suppresses growth of gastric cancer
To further confirm the synergistic inhibitory effects of DY131 and GSK126 on GC growth, the MGC803 cells were subcutaneously implanted into nude mice and treated with 30 mg/kg DY131 or 50 mg/kg GSK126 alone or in combination. After 20 days of treatment, no significant decrease of body weight was observed (Fig. 4a, b), suggesting that the treatments were safe. Also, 30 mg/kg DY131 or 50 mg/kg GSK126 alone significantly suppressed the growth of MGC803 xenograft (Fig. 4c–e). However, the combined inhibitory effect of DY131 and GSK126 was more pronounced, compared to administration of each drug. At the end of treatment, the tumor weight was approximately 0.3 g in separated DY131 and GSK126 groups, while it was only 0.07 g in the combined DY131 and GSK126 group (Fig. 4d, e). Altogether, these data further confirmed that inhibition of EZH2 and activation of ERRγ synergistically suppressed GC.
DY131 and GSK126 synergistically inhibited the growth of GC tumors. a Photographs of nude mice after administration with or without 30 mg/kg of DY131 or/and 50 mg/kg of GSK126 for 20 days. b, c Body weight (b) and tumor volume (c) of nude mice during the administration of DY131 or/and GSK126. d, e Photographs (d) and weight quantification (e) of tumors at the end of DY131 or/and GSK126 treatment. *p < 0.05, ***p < 0.001
Inhibition of EZH2 and activation of ERRγ synergistically inhibits the tumorigenesis of GC cells through FOXM1 signaling pathway
To understand the molecular mechanisms underlying the synergistic inhibitory effects of EZH2 inhibitor and ERRγ agonist, the role of ERRγ target gene, FOXM1, under DY131 and GSK126 treatment was investigated. In MGC803 cells, addition of DY131 or GSK126 alone effectively inhibited the expression of FOXM1, while the effect was much stronger under the combined treatment (Fig. 5a). Consistently, the expression of FOXM1 in MGC803-derived tumors was significantly lower under the combination treatment than each single treatment or control group (Fig. 5b). Importantly, overexpression of FOXM1 greatly blocked the inhibitory effects of GSK126 and DY131 on cell proliferation and migration, wound healing, and colony formation (Fig. 5c–f).
DY131 and GSK126 synergistically inhibit the tumorigenesis of GC cells through FOXM1. a Protein levels of FOXM1 in MGC803 cells under GSK126 or/and DY131 treatment. b Protein levels of FOXM1 in GSK126 and DY131-treated mouse xenograft samples. c–f Cell proliferation (c), wound healing (d), migration (e), and colony formation (f) of MGC803 were detected after treatment with 5 μM DY131 and 10 μM GSK126 in FOXM1-overexpressing cell line. g Protein levels of β-Catenin in MGC803 cells under GSK126 or/and DY131 treatment. h β-Catenin protein levels in GSK126 and DY131-treated mouse xenograft samples. i Protein levels of FOXM1 and β-Catenin in FOXM1- overexpressing MGC803 cells under treatment with GSK126 and DY131. j–m The mRNA levels of PLK1, VEGF, CDC25Bl and MYC were detected by RT-qPCR after treatment of FOXM1-overexpressing cells with 5 μM DY131 and 10 μM GSK126. All experiments were performed in triplicate, and the values given represent the mean ± SD of three independent experiments. Statistical significance was defined as **p < 0.01, ***p < 0.001
Consequently, the signaling pathways regulated by FOXM1 were further investigated when administering a combined DY131 and GSK126 treatment. Previous studies demonstrated that interaction of FOXM1 with β-Catenin promoted β-Catenin nuclear localization and cell proliferation [32]. In both, MGC803 and MGC803-derived tumors, DY131 and GSK126 synergistically decreased β-Catenin protein levels (Fig. 5g, h), mainly in the nucleus (Fig. S9a); meanwhile, overexpression of FOXM1 restored its protein levels under the combined treatment (Figs. 5i, S9a). Furthermore, DY131 and GSK126 synergistically inhibited Top Fish activity (Fig. S9b). Consistently, overexpression of ERRγ or knockdown of EZH2 significantly decreased nuclear β-Catenin protein levels in MGC803 cells, which were restored by FOXM1 overexpression (Fig. S9c).
Four other genes located downstream of FOXM1 were also investigated. As shown in Fig. 5j–m, DY131 and GSK126 synergistically inhibited the expression of PLK1 (Fig. 5j), VEGF (Fig. 5k), CDC25B (Fig. 5l), and MYC (Fig. 5m). Importantly, overexpression of FOXM1 restored the expression of the four genes under combined treatment with DY131 and GSK126 (Fig. 5j–m). Taken together, these data suggested that inhibition of EZH2 and activation of ERRγ synergistically inhibited the expression of FOXM1 and its regulated signaling pathways, which were critical for the synergistic antitumor activity.
Discussion
Gastric cancers are the most common cancers worldwide and the predominant cause of mortality in Asian populations. However, effective targeting therapeutic methods are still lacking. In this study, we showed that EZH2 administration to GC inhibited the expression of ERRγ, which subsequently activated the expression of a master oncogene, FOXM1, and its regulated signaling pathways. Importantly, the combination of EZH2 inhibitor (GSK126) and ERRγ agonist (DY131) significantly suppressed the GC growth.
ERRγ, an orphan nuclear hormone receptor that belongs to the ERR subfamily of transcription factors, is a well-known mediator of mitochondrial biogenesis and cellular energy homeostasis, and has been implicated in many metabolic pathological conditions, such as insulin resistance, alcoholic liver injury, and cardiac hypertrophy [33]. Recently, a few studies showed that ERRγ also played important roles in cancer development. Specifically, ERRγ expression was up-regulated in liver cancer and its inhibition suppressed liver cancer cell proliferation via induction of p21 and p27 [34], while more reports provided evidenced that ERRγ is a tumor suppressor. ERRγ expression was identified in epithelial cell nuclei in fetal and pubertal human prostates, whereas its nuclear expression became reduced in advanced prostate cancer lesions. Furthermore, ERRγ induced E-cadherin, promoted MET, and suppressed breast cancer growth. Overexpression of ERRγ inhibited cell proliferation of prostate cancer cells by arresting cell-cycle progression at the G1-S phase transition [31]. However, ERRγ also mediated the resistance of invasive lobular breast carcinoma to tamoxifen [35], which suggested different roles of ERRγ in different conditions. Importantly, a recent study indicated that the expression of ERRγ was decreased in GC and thus associated with a poor clinical outcome. On the other hand, overexpression of ERRγ suppressed GC cell growth and tumorigenesis via repressing the expression of Wnt signaling genes [19]. In this study, protein levels of ERRγ in 375 GC patient tumor samples were significantly lower than adjacent tissues (Fig. S1c, d) according to immunohistochemistry. Furthermore, ERRγ expression level was positively correlated with the prognosis of GC patients (Fig. S1b, e). Importantly, the overexpression of ERRγ inhibited the tumorigenesis of gastric cancer cells (Fig. S2). Collectively, our data confirmed that ERRγ was a good GC suppressor.
Although ERRγ has been implicated in several types of cancer, the mechanism that regulates its expression is still unclear. In this study, a negative correlation between the ERRγ and EZH2 protein levels was observed (Fig. 1a, b), and EZH2 directly regulated the expression of ERRγ via H3K27me3 (Fig. 1c–f). EZH2 methylated histone methyltransferase H3K27 to promote transcriptional silencing [36]. Many studies have reported links between EZH2 expression and cancers [37], for instance that EZH2 overexpression was associated with progression of prostate cancer [38]. Similar findings were noted in many human cancers, including breast, bladder, and endometrial cancer, melanoma, and GC [39]. Also, high EZH2 expression was correlated with poor prognosis of GC patients and 5-FU resistance [40], and with promoting GC cell proliferation by repressing p21 expression [41]. In this study, we also noticed high expression of EZH2 in GC, and its negative correlation with prognosis of GC patients (Fig. S5). On the other hand, knocking down EZH2 expression inhibited the tumorigenesis of GC cells (Fig. S4). Importantly, we demonstrated that GC suppressor ERRγ was directly regulated by EZH2. Therefore, the combination therapy method targeting ERRγ and EZH2 could be a very promising treatment of GC patients.
FOXM1 is a transcription factor highly expressed in various tumors, which plays a pivotal role in transducing upstream signals to downstream effectors in cancer cells (e.g., cyclin D1, cyclin B1, VEGF, caveolin-1) [42, 43]. More importantly, it is a biomarker and a major predictor of adverse outcomes in 18,000 cancer cases across 39 human malignancies [44]. Almost the complete FOXM1 regulatory mechanism has been identified [45], for instance, Li et al. identified miR-494 as a negative regulator of FOXM1. Specifically, loss of SMAD4 in pancreatic ductal adenocarcinoma cells led to reduced levels of miR-494 and increased levels of FOXM1 [46]. However, the upstream regulators of FOXM1 expression have not yet been elucidated in GC. In this study, we identified two ERRE elements in the proximal promoter sequence of FOXM1 (Fig. 2a). Furthermore, ChIP and EMSA assays demonstrated that ERRγ binding to two ERRE elements on the promoter of FOXM1 regulated transcription of FOXM1 (Fig. 2b, c). Consequently, the combination of GSK126 and DY131 synergistically inhibited the expression of FOXM1 in GC cells and their derived tumors (Fig. 5a, b). Consistently, GSK126 and DY131 inhibited the expression of downstream genes, MYC, PLK1, VEGF, and CDC25B, and decreased β-Catenin protein levels, which was regulated by interaction with FOXM1 (Figs. 5, S9). Thus, ERRγ has been identified as a novel player in regulating FOXM1 mechanism.
Pharmacotherapy can bridge the gap between lifestyle modification and surgery. Although studies have reported that DY131 and GSK126 can inhibit GC proliferation [19, 47], but many monotherapies have only modest efficacy or require high doses with unacceptable side effects. On the other hand, combination therapy is now becoming accepted as a way of optimizing efficacy for tumor management, while minimizing adverse effects [19, 48]. In this study, low concentrations of GSK126 (10 μM) and DY131 (5 μM) successfully treated GC cell lines. GSK126 treatment up-regulated ERRγ protein levels; meanwhile, DY131 activated the function of ERRγ after binding. GSK126 and DY131 administration synergistically inhibited the tumorigenesis of GC cells (Fig. 3). Subsequently, we used 30 mg/kg DY131 and 50 mg/kg GSK126 for mouse studies. To note, GSK126 and DY131 concentrations used in this study were much lower than the previously reported as effective: 200 mg/kg GSK126 and 60 mg/kg DY131 [15, 19]. However, the combined treatment achieved better efficacy (Fig. 4). Usually, decreased concentration of the combined drug is beneficial as it reduces the toxicity and synergistically enhances the drug antitumor activity [28]. Presently, few options for combination therapy of GC patients are available. Combination of S-1 plus cisplatin with peritoneal or trastuzumab showed a good performance in GC treatment [49]. However, more targeted chemotherapy strategies are necessary to improve the survival rate of GC patients, and thus drug combination (GSK126 and DY131) is promising.
In summary, we obtained both experimental and clinical evidence supporting the critical role of EZH2 inhibition and ERRγ activation in synergistically suppressing GC via inhibiting FOXM1 signaling pathways (Fig. 6). First, the expression of ERRγ and EZH2 were inversely correlated in GC specimens and cell lines. Second, ERRγ expression was markedly negative-regulated by EZH2 in GC cell lines. Third, ERRγ acted as a direct suppressor of FOXM1. Fourth, inhibition of EZH2 (GSK126) and activation of ERRγs (DY131) synergistically induced an antitumor activity in GC via FOXM1 signaling pathway. Collectively, these novel findings identified a novel pathway regulating the growth of gastric tumor, in which EZH2 suppressed the expression of ERRγ, subsequently activating the expression of FOXM1.
Schematic diagram of the synergistic inhibition of GC by GSK126 and DY131. FOXM1 promoted proliferation by promoting MYC, and expression and nuclear localization of β-Catenin in GC cells. GSK126 inactivated EZH2 and subsequently promoted the ERRγ expression. DY131 activated ERRγ. The combination of GSK126 and DY131 activated the expression and function of ERRγ, and synergistically inhibited the expression of FOXM1 and downstream pathways, thereby inhibiting the proliferation of GC cells
References
Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–13.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386.
Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;64:1881–8.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Lee SY, Oh SC. Changing strategies for target therapy in gastric cancer. World J Gastroenterol. 2016;22:1179–89.
Parisa Karimi FI, Sharmila A, Freedman ND, Kamangar F. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–53.
Camargo MC, Figueiredo C, Machado JC. Review: Gastric malignancies: basic aspects. Helicobacter. 2019;24(1):e12642.
de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am. 2013;42:219–40.
Horvat A, Noto JM, Ramatchandirin B, Zaika E, Palrasu M, Wei J, et al. Helicobacter pylori pathogen regulates p14ARF tumor suppressor and autophagy in gastric epithelial cells. Oncogene. 2018;37:5054–65.
Hu Y, Liu JP, Li XY, Cai Y, He C, Li NS, et al. Downregulation of tumor suppressor RACK1 by Helicobacter pylori infection promotes gastric carcinogenesis through the integrin beta-1/NF-kappaB signaling pathway. Cancer Lett. 2019;450:144–54.
Holokai L, Chakrabarti J. Increased programmed death-ligand 1 is an early epithelial cell response to Helicobacter pylori Infection. PLoS Pathog. 2019;15:e1007468.
Coker OO, Dai Z, Nie Y, Zhao G, Cao L, Nakatsu G, et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut. 2018;67:1024–32.
Hsieh YY, Tung SY, Pan HY, Yen CW, Xu HW, Lin YJ, et al. Increased abundance of Clostridium and Fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Sci Rep. 2018;8:158.
Petrovchich I, Ford JM. Genetic predisposition to gastric cancer. Semin Oncol. 2016;43:554–9.
Li J, Woods SL, Healey S, Beesley J, Chen X, Lee JS, et al. Point mutations in exon 1B of APC reveal gastric adenocarcinoma and proximal polyposis of the stomach as a familial adenomatous polyposis variant. Am J Hum Genet. 2016;98:830–42.
van der Post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, Hoogerbrugge N, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52:361–74.
Network TR. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9.
Rocken C. Molecular classification of gastric cancer. Expert Rev Mol Diagn. 2017;17:293–301.
Kang MH, Choi H, Oshima M, Cheong JH, Kim S, Lee JH. Estrogen-related receptor gamma functions as a tumor suppressor in gastric cancer. Nat Commun. 2018;9:1920.
Padmanabhan N, Ushijima T, Tan P. How to stomach an epigenetic insult: the gastric cancer epigenome. Nat Rev Gastroenterol Hepatol. 2017;14:467–78.
Waddell T, Chau I, Cunningham D, Gonzalez D, Frances A, Okines C, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481–9.
Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–9.
Bang YJ, Van Cutsem E, Feyereislova A, Investigators TT. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (TOGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:1302–1302.
Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–76.
Leiting JL, Grotz TE. Advancements and challenges in treating advanced gastric cancer in the West. World J Gastrointest Oncol. 2019;11:652–64.
Xu S, Adisetiyo H, Tamura S, Grande F, Garofalo A, Roy-Burman P, et al. Dual inhibition of survivin and MAOA synergistically impairs growth of PTEN-negative prostate cancer. Br J Cancer. 2015;113:242–51.
Kim JS, Turbov J, Rosales R, Thaete LG, Rodriguez GC. Combination simvastatin and metformin synergistically inhibits endometrial cancer cell growth. Gynecol Oncol. 2019;154:432–40.
Kurokawa Y, Sugimoto N, Miwa H, Tsuda M, Nishina S, Okuda H, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer. 2014;110:1163–8.
Yamashita-Kashima Y, Iijima S, Yorozu K, Furugaki K, Kurasawa M, Ohta M, et al. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res. 2011;17:5060–70.
Audet-Walsh E, Yee T, McGuirk S, Vernier M, Ouellet C, St-Pierre J, et al. Androgen-dependent repression of ERRgamma reprograms metabolism in prostate cancer. Cancer Res. 2017;77:378–89.
Yu S, Wang X, Ng CF, Chen S, Chan FL. ERRgamma suppresses cell proliferation and tumor growth of androgen-sensitive and androgen-insensitive prostate cancer cells and its implication as a therapeutic target for prostate cancer. Cancer Res. 2007;67:4904–14.
Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, et al. FoxM1 promotes beta-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell. 2011;20:427–42.
Misra J, Kim DK, Choi HS. ERRgamma: a junior orphan with a senior role in metabolism. Trends Endocrinol Metab. 2017;28:261–72.
Kim JH, Choi YK, Byun JK, Kim MK, Kang YN, Kim SH, et al. Estrogen-related receptor gamma is upregulated in liver cancer and its inhibition suppresses liver cancer cell proliferation via induction of p21 and p27. Exp Mol Med. 2016;48:e213.
Riggins RB, Lan JPJ, Klimach U, Zwart A, Cavalli LR, Haddad BR, et al. ERR gamma mediates tamoxifen resistance in novel models of invasive lobular breast cancer. Cancer Res. 2008;68:8908–17.
Di Croce L, Helin K. Transcriptional regulation by Polycomb group proteins. Nat Struct Mol Biol. 2013;20:1147–55.
Yamagishi M, Uchimaru K. Targeting EZH2 in cancer therapy. Curr Opin Oncol. 2017;29:375–81.
Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–9.
Kim KH, Roberts CW. Targeting EZH2 in cancer. Nat Med. 2016;22:128–34.
Wang C, Li X, Zhang J, Ge Z, Chen H, Hu J. EZH2 contributes to 5-FU resistance in gastric cancer by epigenetically suppressing FBXO32 expression. Onco Targets Ther. 2018;11:7853–64.
Xu JW, Wang Z, Lu W, Jiang H, Lu J, Qiu J, et al. EZH2 promotes gastric cancer cells proliferation by repressing p21 expression. Pathol Resh Pract. 2019;2019:215.
Gartel AL. FOXM1 in cancer: interactions and vulnerabilities. Cancer Res. 2017;77:3135–9.
Wang Z, Ahmad A, Li Y, Banerjee S, Kong D, Sarkar FH. Forkhead box M1 transcription factor: a novel target for cancer therapy. Cancer Treat Rev. 2010;36:151–6.
Nandi D, Cheema PS, Jaiswal N, Nag A. FoxM1: Repurposing an oncogene as a biomarker. Semin Cancer Biol. 2018;52:74–84.
Liao GB, Li XZ, Zeng S, Liu C, Yang SM, Yang L, et al. Regulation of the master regulator FOXM1 in cancer. Cell Commun Signal. 2018;16:57.
Li L, Li Z, Kong X, Xie D, Jia Z, Jiang W, et al. Down-regulation of microRNA-494 via loss of SMAD4 increases FOXM1 and beta-catenin signaling in pancreatic ductal adenocarcinoma cells. Gastroenterology. 2014;147:485–497.e418.
Chen YT, Zhu F, Lin WR, Ying RB, Yang YP, Zeng LH. The novel EZH2 inhibitor, GSK126, suppresses cell migration and angiogenesis via down-regulating VEGF-A. Cancer Chemother Pharmacol. 2016;77:757–65.
Lin J, Wu L, Bai X, Xie Y, Wang A, Zhang H, et al. Combination treatment including targeted therapy for advanced hepatocellular carcinoma. Oncotarget. 2016;7:71036–51.
Hara T, Fujiwara Y, Sugimura K, Omori T, Motoori M, Miyoshi N, et al. S-1 plus cisplatin combination therapy for gastric cancer with peritoneal dissemination. Gan To Kagaku Ryoho. 2015;42:1466–8.
Acknowledgements
This work was supported by the Natural Science Foundation of Guangdong Province (2015A030312005), the Science and Technology Program of Guangzhou (201804020067) and the Department of Education of Guangdong Province (2017KCXTD001, 2018KZDXM015).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving animals and informed consent
All samples were obtained with patients’ informed consent. The Ethics Committee of Sun Yat-sen University Cancer Center approved this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, B., Mu, P., Yu, Y. et al. Inhibition of EZH2 and activation of ERRγ synergistically suppresses gastric cancer by inhibiting FOXM1 signaling pathway. Gastric Cancer 24, 72–84 (2021). https://doi.org/10.1007/s10120-020-01097-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-020-01097-x