Abstract
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract; most of them have gain-of-function mutations of the KIT gene. There have been rare cases of families with multiple GISTs, that had autosomal dominant germline KIT mutations. Here, we present a case of multiple GISTs caused by a novel germline KIT mutation. Intraoperatively, the main tumor was present in the body of the stomach, and multiple small nodules were detected mainly in the upper and middle part of the gastric wall; several nodules were also present in the small bowel wall. The main tumor and surrounding nodules were resected. DNA sequencing of the tumor tissue, adjacent normal mucosal tissue, and peripheral blood leukocytes revealed that the patient had germline Asp820Gly mutation in exon 17 of the KIT gene. This is the first case with germline Asp820Gly mutation in exon 17 of the KIT gene.
Similar content being viewed by others
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract [1]. GISTs are derived from the interstitial cells of Cajal (ICCs) or their precursors owing to gain-of-function mutations in either the KIT gene that encodes the KIT receptor tyrosine kinase (CD117), or the platelet-derived growth factor receptor alpha gene [2]. Most GISTs are sporadic, and often have mutations in exons 11 or 9 of the KIT gene. In contrast, familial and multiple GISTs with germline KIT mutations occur as a rare autosomal dominant disorder. Approximately 40 cases of familial GIST have been reported to date [3]. Mutations in exon 11 of the KIT gene were detected in 25 families, while mutations in exon 17 were detected in only four families. The previously observed mutations in exon 17 are Asp820Tyr and Asn822Tyr [4,5,6,7]. Herein, we present the case of a patient with multiple GISTs with a novel germline KIT gene mutation (Asp820Gly) in exon 17.
Case presentation
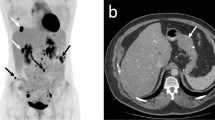
In 2009, a 73-year-old female patient was admitted to the Department of Gastrointestinal Medicine at our hospital due to epigastric pain. She had no symptoms on the skin such as neurofibromas and café-au-lait spots, reminiscent of type 1 neurofibromatosis. Upon endoscopic examination, multiple gastric submucosal tumors (SMTs) were found in the lesser curvature of the stomach (Fig. 1a). The patient was followed up as an outpatient as she did not have severe symptoms, and her SMTs were of a moderate size (diameter < 2 cm). Gastric endoscopic examinations were performed in 2009, 2010, and 2012. In 2010, the patient started experiencing heartburn, and the maximum diameter of the SMTs on computed tomography (CT) scan was approximately 2 cm (Fig. 1b). However, in 2015, surgical treatment was required owing to enlargement of the tumors to > 5 cm in diameter (Fig. 1c, d).
Representative images obtained on endoscopic examination and computed tomography (CT) scan. a Endoscopic image showing multiple SMTs in 2009 (white arrowheads). b A CT image of the main tumor in 2010. The maximum diameter was approximately 2 cm (red arrowheads). Scale bar: 5 cm. c An endoscopic image of the main tumor in 2015 (white arrowheads). d A CT image of the main tumor in 2015. The maximum diameter was greater than 5 cm (red arrowheads). Scale bar: 5 cm
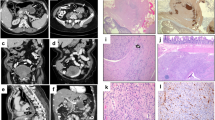
Intraoperatively, the main tumor was found in the body of the stomach (Fig. 2a). Multiple small nodules were also detected, mainly in the upper and middle part of the gastric wall (Fig. 2b), and several nodules were observed in the small bowel (Fig. 2c). Considering the treatment burden related to surgery, only the main tumor and the nodules around the main tumor were resected.
Results
The operative specimens revealed that the main tumor had a diameter of 8 cm (Fig. 3a, b). Histopathological examination showed that multiple small nodules were present around the main tumor, which along with the small surrounding nodules were composed of spindle shaped cells (Fig. 3c). The tumor cells tested positive for both, KIT and CD34 on immunohistochemical examination (Fig. 3d, e), and the mitotic rate was less than 5 per 50 high-power fields. The risk stratification of the main tumor was intermediate, according to the modified Fletcher classification. Sequence analysis of the genomic DNA revealed that the main tumor had an Asp820Gly mutation in exon 17 of the KIT gene (Fig. 4). The mutation was also detected in the adjacent normal mucosal tissue of the stomach, indicating that it was a germline mutation (Fig. 4). Sequence analysis using genomic DNA derived from peripheral leukocytes confirmed the germline Asp820Gly mutation (Fig. 4).
Macroscopic and histological images of operative specimens. a A macroscopic image of the resected main tumor. b The cut surface of the tumor. The maximum diameter of the main tumor was 8 cm, including the necrotic tissue. c Specimens stained with hematoxylin and eosin showed that the tumor was composed of proliferation of spindle shaped cells (original magnification: × 200). d Immunostaining for KIT showed positive findings (original magnification: × 40). e Immunostaining for CD34 also showed positive findings (original magnification: × 40)
The results of the DNA sequence analysis of the KIT gene in exon 17. Adenine has been replaced by guanine heterozygously at codon 820 of exon 17, resulting in the exchange of aspartic acid (Asp) for glycine (Gly), Asp820Gly, in the patient’s samples. The upper, middle, and lower DNA sequences indicate the main tumor, normal mucosal tissue, and peripheral leukocytes, respectively
Discussion
In familial and multiple GISTs, various types of germline mutations have been detected in exons 8, 11, 13, and 17. Approximately 40 cases of familial and multiple GIST with germline KIT mutation have been reported to date [3], among which 25, 9, and 4 families had mutations of exons 11, 13, and 17, respectively. Only one case each has been reported with familial and multiple GIST with mutations in exons 8 or 9. The present case is the fifth report of a case involving multiple GISTs having an exon 17 KIT mutation. Notably, this is the first case of an Asp820Gly germline mutation, since previously detected exon 17 mutations included only Asp820Tyr and Asn822Tyr (Table 1) [4,5,6,7].
Some concomitant symptoms of familial GISTs have been reported. Since the function of the KIT receptor tyrosine kinase is essential for the development of both, ICCs and melanocytes and mast cells, gain-of-function mutations of the KIT gene may induce diffuse ICC hyperplasia, hyperpigmentation, and mastocytoma. ICC hyperplasia at the gastroesophageal junction may be associated with achalasia-like dysphagia. Heartburn, a symptom observed in the present case, may be related to the dysphagia caused by ICC hyperplasia at the gastroesophageal junction [5].
We experienced a case of multiple GISTs caused by a novel germline KIT gene mutation (Asp820Gly); this patient was probably a proband with the novel mutation. The diagnostic criteria for familial GISTs have not been formally defined yet. The detection of germline KIT gene mutations in both, tumor tissues and adjacent normal tissue or blood samples (peripheral leukocytes) is usually necessary for a positive diagnosis. In the present case, although we demonstrated the presence of mutations in the all the samples, i.e., tumor tissues, adjacent normal mucosal tissue, and peripheral leukocytes (Fig. 4), further investigations into detailed family history, including germline mutations, are needed to prove familial GIST.
References
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80.
Vale Rodrigues R, Santos F, Pereira J, da Silva I, Claro FI, Albuquerque C, et al. A case of multiple gastrointestinal stromal tumors caused by a germline KIT gene mutation (p.Leu576Pro). Fam Cancer. 2017;16:267–70.
Sekido Y, Ohigashi S, Takahashi T, Hayashi N, Suzuki K, Hirota S. Familial gastrointestinal stromal tumor with germline kit mutations accompanying hereditary breast and ovarian cancer syndrome. Anticancer Res. 2017;37:1425–31.
Thalheimer A, Schlemmer M, Bueter M, Merkelbach-Bruse S, Schildhaus HU, Buettner R, et al. Familial gastrointestinal stromal tumors caused by the novel KIT exon 17 germline mutation N822Y. Am J Surg Pathol. 2008;32:1560–5.
Hirota S, Nishida T, Isozaki K, Taniguchi M, Nishikawa K, Ohashi A, et al. Familial gastrointestinal stromal tumors associated with dysphagia and novel type germline mutation of KIT gene. Gastroenterology. 2002;122:1493–9.
O'Riain C, Corless CL, Heinrich MC, Keegan D, Vioreanu M, Maguire D, et al. Gastrointestinal stromal tumors: insights from a new familial GIST kindred with unusual genetic and pathologic features. Am J Surg Pathol. 2005;29:1680–3.
Veiga I, Silva M, Vieira J, Pinto C, Pinheiro M, Torres L, et al. Hereditary gastrointestinal stromal tumors sharing the KIT Exon 17 germline mutation p.Asp820Tyr develop through different cytogenetic progression pathways. Genes Chromosomes Cancer. 2010;49:91–8.
Acknowledgements
We are grateful to the staff of the Department of Surgical Pathology, Hyogo College of Medicine Hyogo, Japan, for analyzing the samples. We would also like to thank Editage (https://www.editage.jp) for English language editing.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Patient anonymity and informed consent
Written informed consent was obtained from the patient for publication of this report and the accompanying images, and the patient’s anonymity was upheld.
Availability of data and materials
All data supporting the conclusions of this study are included in this published article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arima, J., Hiramatsu, M., Taniguchi, K. et al. Multiple gastrointestinal stromal tumors caused by a novel germline KIT gene mutation (Asp820Gly): a case report and literature review. Gastric Cancer 23, 760–764 (2020). https://doi.org/10.1007/s10120-020-01055-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-020-01055-7