Abstract
Background
To safely perform minimized gastrectomy based on sentinel node (SN) concept for early gastric cancer patients, intraoperative diagnostic accuracy is indispensable. This study aimed to evaluate the clinical utility of the one-step nucleic acid amplification (OSNA) assay in the intraoperative diagnosis of SN metastasis in early gastric cancer patients compared with that of histopathological examination.
Methods
We conducted a prospective study using the OSNA assay for 43 patients with cT1N0M0 gastric cancer undergoing gastrectomy with SN mapping. All the SNs and selected non-SNs were examined by routine histopathological diagnosis, and the OSNA assay.
Results
We performed permanent histopathology (PH) in 1732 lymph nodes (LNs) (286 SNs and 1446 non-SNs) obtained from 43 patients. We also evaluated 439 LNs (286 SNs and 153 non-SNs) with the OSNA assay in addition to PH. Intraoperative histopathology (IH) was performed in 214 LNs (213 SNs and 1 non-SN). PH revealed LN metastasis in 6 patients (14%), all of whom showed positive SNs by PH. The diagnostic accuracy to predict the LN status based on the SN concept by histological examination was 100%. The concordance rate between the OSNA assay and the PH and IH were 0.970 and 0.981 respectively. Discordant results between PH and OSNA assay were observed in 13 LNs. The sensitivity and specificity of the OSNA assay compared with those of PH were 0.636, and 0.988, and compared with those of IH were 0.800, and 0.995.
Conclusion
Our results suggest that the OSNA assay is a useful and convenient tool for the intraoperative detection of SN metastasis in early gastric cancer patients.
Similar content being viewed by others
Introduction
Sentinel node (SN) is defined as the first draining lymph node (LN) that receives the lymphatic flow directly from a primary tumor [1]. According to the SN concept, SN is the first LN that the metastasis emerges from. SN concept is already accepted in the field of melanoma and breast cancer [1,2,3]. Recently, we have published the results of a multicenter prospective study showing favorable results of SN mapping in terms of detection rate and accuracy to determine the LN status in early gastric cancer patients [4]. LN metastases are commonly detected using hematoxylin and eosin (H&E) staining of a section that includes the largest dimension of the LN [4, 5]. To safely apply minimized gastrectomy based on the results of SN mapping, the accurate detection of LN metastasis including micrometastasis during surgery is indispensable. In our previous report, we reported the accuracy of the diagnosis of intraoperative histopathology (IH) using frozen sections to be about 70% [4]. Compared to H&E staining, a molecular biological approach, such as reverse transcription polymerase chain reaction (RT-PCR), is thought to be more accurate in the diagnosis of micrometastasis, because it allows the examination of the whole LNs [6]. Multiplex RT-PCR method had also been reported to be suitable for the intraoperative detection of micrometastases [7]. However, it is too time consuming to apply these methods for the diagnosis of intraoperative SN metastasis. On behalf of RT-PCR procedure, a one-step nucleic acid amplification (OSNA) assay, which is already being used in field of breast cancer [8], is thought to be an attractive alternative diagnostic tool for the diagnosis of SN metastases in gastric cancer patients [9].
OSNA is an automated system using a reverse transcription loop-mediated isothermal amplification (RT-LAMP) method for gene amplification that was developed by Sysmex Corp (Kobe Japan). In this system, the mRNA of the molecular marker cytokeratin 19 (CK19) is directly and rapidly amplified from the supernatant of homogenized LNs [9]. The mRNA purification process that is usually performed in RT-PCR methods is not required in this system. Results are available in 30 per one LN and 50 min per 4 LNs. Yaguchi et al. have defined the CK19 mRNA cutoff value when using the OSNA assay to detect LN metastases in gastric cancer patients [9]. Kumagai et al. reported the utility of the OSNA assay in the diagnosis of the LNs in advanced gastric cancer patients [10]. We considered that if this method can be applied in the diagnosis of SN metastasis, it may ensure the accuracy of intraoperative SN mapping in early gastric cancer patients, and we hypothesized that the OSNA assay is useful in the intraoperative diagnosis of SN mapping for early gastric cancer. The aim of this study was to evaluate the diagnostic accuracy, sensitivity, and specificity of the OSNA assay compared to that of the histological examination in the SN assessment for early gastric cancer patients.
Patients and methods
Patients
We analyzed 43 patients with cT1N0M0 early gastric cancer who underwent gastrectomy with SN mapping between 2009 and 2011 in Keio University Hospital, Japan. Clinical staging was made by preoperative endoscopy and computed tomography, in accordance with the Japanese Classification of Gastric Cancer proposed by the Japanese Gastric Cancer Association and according to the TNM classification [11, 12].
Methods
SN biopsy
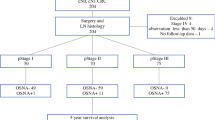
We used radioactive colloid and blue dye (0.5% indocyanine green or 1% blue dye) as a tracer, as described previously [5]. Briefly, the day before the surgery, we injected 2.0 ml (150 MBq) of a solution of a technetium-99 m tin colloid into the 4 quadrants of the submucosal layer surrounding the primary tumor using an endoscopic puncture needle. During the surgery, we injected 0.5% indocyanine green (ICG) or 1% isosulfan blue as a blue dye by the same method we used for the radioactive colloid. We defined SNs as LNs with radioactivity of > 10-times the background activity measured by handheld gamma probe or LNs with blue stained. All the detected SNs and randomly selected non-SNs were evaluated in this study. In patients who had function-preserving minimized gastrectomy, all the non-SNs belonged to the SN basins. On the other hand, in patients who had standard gastrectomy, the non-SNs belonged to both SN and non-SN basins. SNs and selected non-SNs were divided into 2 pieces, and half of the pieces were subjected to the OSNA assay (Block A). The other half of the pieces (Block B) was subjected to the intraoperative histopathologic diagnosis, which was confirmed in permanent histological sections (Fig. 1). We considered function-preserving surgery in patients with no SN metastasis. The concordance rate between the two methods was evaluated to confirm the accuracy of the OSNA assay. We also compared the sensitivity and the specificity of the OSNA assay with those of the histological H&E staining method. In addition, we performed the quantitive RT-PCR (qRT-PCR) using the rest of the solubilized liquid after performing the OSNA assay.
We further analyzed the LNs with discordant results between the OSNA assay and the histopathological examination. To confirm the presence of metastasis, we sliced the Block B specimen into several 0.2 mm pieces, and stained them with H&E as well as anti-CK19 antibody (RCK108; DAKO, Glostrup, Denmark). A pathologist then identified the presence of metastasis in these slides based on both results of the H&E and immunostaining.
This study was approved by the Institutional Review Board of Keio University School of Medicine. All the patients were given a written informed consent for the entire procedure of SN mapping before the surgery (IRB approval number: 2009-90).
OSNA assay
CK19 mRNA was used as a marker in the OSNA assay. Block A was homogenized using LYNORHAG lysis buffer (Sysmex, Kobe, Japan) and centrifuged at 10,000×g. LYNOAMP BC gene amplification reagent (Sysmex) was added to the supernatant, and CK19 mRNA in each lysate was amplified and detected in the RD-100i system (Sysmex), an automated molecular detection system that uses RT-LAMP method [13]. This method measures the time taken to exceed a predetermined threshold turbidity caused by magnesium pyrophosphate, a byproduct of the amplification reaction. Amplification times were analyzed based on a previously generated standard curve, and the results of the assay were expressed as the number of CK19 mRNA copies per microliter (copies/µl). In this study, we used the cutoff value of 250 copies/µl, based on a previous study [9].
qRT-PCR
We performed qRT-PCR by the same methods that Tsujimoto et al. reported in breast cancer patients previously [14]. qRT-PCR was carried out by ABI Prism 7700 sequence detector. RNA was purified from a LN lysate using RNeasy Mini Kit (Qiagen), and the purified RNA was subjected to one-step RT-PCR with Quanti Tect SYBR Green (Qiagen) according to the manufacturer’s instructions. We designed the primers using the Primer Express software version 2.0 (ABI) [14].
Statistical analysis
We performed the statistical analyses using SPSS software, version 22 (IBM Corporation, Armonk, NY, USA). We analyzed the clinical and pathological variables using the χ2 test and Fisher’s exact tests. We considered differences to be statistically significant at p < 0.05.
Results
We performed histological examination in 1732 LNs (286 SNs and 1446 non-SNs) from 43 patients, and IH in 214 LNs (213 SNs and 1 non-SN). We also evaluated 439 LNs (286 SNs and 153 non-SNs) using histological examination and OSNA assay (Fig. 1). The patients’ characteristics are described in Table 1.
Table 2 shows the SN status according to the permanent histopathology (PH), IH, and OSNA assay. Histological LN metastasis was revealed in 6 of 43 patients (14%) (Table 2a, c), all of whom showed positive SNs by histological examination. The sensitivity and overall accuracy of the SN mapping were 100% each, with no false negative cases (Table 2a–c).
Table 3 shows the results of comparison of PH and the OSNA assay (3a), and of IH and the OSNA assay (3b). There were 13 LNs out of 9 patients that showed discordant results between the OSNA assay and PH (3a). The concordance rate between the OSNA assay and the histological examination of paraffin specimens was 0.970 (426/439) (95% Confidence interval (CI), 0.950–0.984). The sensitivity and specificity of the OSNA assay compared with those of PH were 0.636 (14/22) (95% CI, 0.407–0.828) and 0.988 (412/417) (95% CI, 0.972–0.996), respectively. The OSNA assay had a concordance rate of 0.981(210/214) (95% CI, 0.953–0.995), sensitivity of 0.800 (12/15) (95% CI, 0.519–0.957), and specificity of 0.995 (198/199) (95% CI, 0.972–1.000) with IH diagnoses of frozen sections.
We further evaluated the 13 discordant LNs (3.0%) from 9 patients, 2 of which were too small to be cut into a thin slice; therefore, we evaluated the remaining 11 LNs. We made thin slides for H&E and immunostaining using anti-CK19 antibody. Our pathologist comprehensively determined the metastasis status in the deep cut slices according to the results of H&E staining and anti-CK19 antibody immunohistochemistry. There were 3 LNs (one SN and 2 non-SNs) from 5 patients with PH (−) OSNA (+), and 1 (non-SN) of them was positive for metastasis in the deep cut slides. On the other hand, there were 8 LNs (SNs) from 4 patients with PH (+) OSNA (−), and 3 of them were found to have no metastasis, 3 had micrometastasis (0.2 mm, 0.7 mm and 1.5 mm in size), and 2 had macrometastasis (3.0 mm and 3.1 mm in size) in the deep cut slides. The histological pathology and pathological tumor depth of these patients were as follows: tubular adenocarcinoma/poorly adenocarcinoma/signet ring cell carcinoma; 1/4/4, and T1a/T1b/T2/T3; 5/4/0/0. The pathology of all the patients with PH (+) OSNA (−) were poorly adenocarcinoma/signet ring cell carcinoma.
With regard to the LN metastasis, there were 9 of the 43 patients who had LN metastasis diagnosed by at least one technique. LN metastases in 7 patients (78%) were diagnosed by the OSNA assay. We found no significant differences in terms of clinical features between patients with metastases (n = 7) and those without (n = 36), based on the OSNA assays (Table 4).
Figure 2 shows the results of CK19 mRNA levels detected by the OSNA assay and qRT-PCR. Our results revealed that the CK19 levels were close to the cutoff value in 8 LNs with OSNA-positive and histopathologically-negative LNs, and also very low in the qRT-PCR assay.
Discussion
According to the results of our study, the sensitivity and overall accuracy of SN mapping were both 100% with no false negative cases. This suggests that the SN concept could be safely applied in our study.
Since the SN concept is affordable for patients with early gastric cancer, we expected that the OSNA assay would also play a major role in the diagnosis of SNs in these patients in the near future. The OSNA assay can be applied to assess LN metastasis in a variety of cancers expressing CK19. The clinical utility of the OSNA assay has been reported for several malignant tumors [15]. As for breast cancer, the OSNA assay can be used for the diagnosis of metastasis in SNs [8, 16]. Compared to breast cancer, there are relatively high proportions of undifferentiated types of cancer in gastric cancer patients, which makes it more difficult to validate the OSNA assay in gastric cancer field.
Yaguchi et al. reported the results of the OSNA assay for the diagnosis of LN metastasis in gastric cancer patients [9]. They reported that when they set the cutoff value of CK19 mRNA to be 250 copies/µl, the concordance rate between the OSNA assay and H&E staining would be 94.4%, and the sensitivity and specificity were 88.9 and 96.8% respectively. Kumagai et al. reported the results of a multicenter study to validate the utility of the OSNA assay in detecting LN metastasis in gastric cancer patients [10]. Shoji et al. reported the feasibility and safety of a single-tracer SN mapping by ICG fluorescence imaging with intraoperative diagnosis by the OSNA assay [17]. However, our study is the first study to evaluate the utility of OSNA assay in the SN assessment in gastric cancer patients which compare the results of OSNA assay with those of histopathological examination. The OSNA assay can facilitate a highly accurate detection of LN metastasis in gastric cancer patients quickly and easily.
In our study, we evaluated not only the LN situation of SNs but also non-SNs. In clinical practice of sentinel node navigation surgery (SNNS), we do not usually evaluate non-SNs intraoperatively. However, we evaluated non-SNs in addition to SNs in our study to demonstrate the accuracy of the OSNA assay in as many LNs as possible. By evaluating SNs and non-SNs we could evaluate 1732 LNs in total. In our study, the OSNA assay showed favorable results not only for SNs but also for non-SNs. Our results showed a high concordance between OSNA and histopathology and we consider the OSNA assay to be a useful, convenient and an objective alternative to the current pathological method of detecting LN metastases. Although SNNS for early gastric cancer is only performed in specialized institutions, we hope SNNS for early gastric cancer may be applied worldwide in the future together with the application of the OSNA assay.
There were 13 discordant results between the OSNA assay and the histological examination in our study. 5 LNs were histologically negative but positive in the OSNA assay, and 8 LNs were histologically positive but negative in the OSNA assay. In those LNs, we used the remaining samples used for histological examination for further study. We made the 0.2 mm-interval serial sections and we found metastasis to be absent in 2 OSNA positive and histologically negative LNs. This may be explained by the tissue allocation bias of CK19 protein expression. Because LN blocks used for the OSNA assay and the histological examination were different, the metastasis may be localized in either of the blocks. Mc Grath et al. reported that examination of three section levels from paraffin blocks containing LN tissue detected more metastatic deposits than examination of one section level only [18]. We also found some cases with negative OSNA assay and higher expression of qRT-PCR. This may also be explained by the tissue allocation bias; for instance, the expression of CK19 might be negative in the specimen used in the OSNA assay.
Yaguchi et al. reported that gastric cancer tissues have been shown to express CK19 at a high rate (98.6%), however, a small proportion of cases do not express CK19 (1.4%) [9]. We found 3 of 8 LNs with OSNA negative and histologically positive LNs were negative with LN metastasis according to the result of the H&E staining and anti-CK19 antibody immunohistochemistry in the deep cut slides. This may be because CK19 expression was low in the 3 negative LNs. Kumagai et al. also reported some cases with low CK19 expression, accounting for 3.3% of all cases [10]. Our study showed that CK19 levels were close to the cutoff value in samples with OSNA positive and histologically negative LNs, and also the low levels were confirmed by the results of the qRT-PCR assay. This result also supports the fact that there are some LNs with a low expression of CK19 which may be difficult to detect with the OSNA assay. This may also be due to the size of the metastatic site. Among the 8 LNs of OSNA negative and histologically positive cases, we found 3 LNs to have no metastasis in the deep cut slides. In the remaining 5 LNs, we found 3 LNs to have micrometastasis (MM), and 2 LNs to have macrometastasis. If the metastatic site is small, it may be difficult to detect the metastasis by using the anti-CK19 antibody immunohistochemistry alone. The clinical significance of MM remains unclear, and therefore, OSNA assay is believed to be useful to detect the possibility of MM. Our results showed a high concordance between OSNA and histopathology. Thus, we consider the OSNA assay to be a useful, convenient and an objective alternative to the current pathological method for detecting LN metastases. However, the diagnostic accuracy of OSNA assay was not 100%, and we cannot completely exclude the possibility of false negative cases caused by MM even if we use OSNA assay. Therefore, we think SN basin dissection is required for all cases to secure oncological safety when we perform SNNS for patients with early gastric cancer.
One of the limitations of this study is that we used different slices of LNs for the OSNA assay and histological examination. The allocation bias is inevitable in our study which could also have affected the results. In addition, about half of the cases were poorly differentiated adenocarcinoma or signet ring cell. The pathology of 8 out of 9 patients with discordant results between OSNA assay and histological examination were poorly differentiated adenocarcinoma or signet ring cell, and especially the pathology of all patients with PH (+) OSNA (−) were poorly differentiated adenocarcinoma or signet ring cell. In addition to the allocation bias, there may be a difference for the diagnostic ability according to the histological pathology that might have affected to these discordant results. Kumagai et al. reported that while there were no significant difference, the discordant results between PH and OSNA were more often seen in patients with undifferentiated-type of tumor [10]. In comparison with our results, it is possible that CK19 expression may be low in some patients with undifferentiated-type of cancer, which makes it difficult for us to detect by OSNA assay. Therefore, we suggest that when we perform OSNA assay in gastric cancer patients, we might be careful to avoid the possibility of false negative results especially in patients with an undifferentiated-type of tumor.
Third, there were 8 out of 43 patients who previously had endoscopic treatment. It is concerned that lymphatic flow may be reconstructed by the endoscopic treatment, however, we have reported that we may safely apply SNNS for patients post endoscopic treatment [19, 20].
Our study showed favorable results for the OSNA assay in the diagnosis of SNs in patients with early gastric cancer. In a future study the whole SN should be examined by the OSNA assay in gastric cancer patients. As the intraoperative diagnostic accuracy is important factor to safely perform minimized gastrectomy in early gastric cancer patients, the OSNA assay can be indispensable.
References
Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–9.
Wong JH, Cagle LA, Morton DL. Lymphatic drainage of skin to a sentinel lymph node in a feline model. Ann Surg. 1991;214:637–41.
Guiliano AE, Kirigan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–8 (Discussion 398–401).
Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704–10.
Takeuchi H, Kitagawa Y. New Sentinel node mapping technologies for early gastric cancer. Ann Surg Oncol. 2013;20:522–32.
Arigami T, Natsugoe S, Uenosono Y, Mataki Y, Ehi K, Higashi H, et al. Evaluation of sentinel node concept in gastric cancer based on lymph node micrometastasis determined by reverse transcription-polymerase chain reaction. Ann Surg. 2006;243:341–7.
Shimizu Y, Takeuchi H, Sakakura Y, Saikawa Y, Nakahara T, Mukai M, et al. Molecular detection of sentinel node micrometastases in patients with clinical N0 gastric carcinoma with real-time multiplex reverse transcription-polymerase chain reaction assay. Ann Surg Oncol. 2012;19:469–77.
Tamaki Y, Akiyama F, Iwase T, Kaneko T, Tsuda H, Sato K, et al. Molecular detection of lymph node metastasis in breast cancer patients: results of a multicenter trial using the one-step nucleic acid amplification assay. Clin Cancer Res. 2009;15:2879–84.
Yaguchi Y, Sugasawa H, Tsujimoto H, Takata H, Nakabayashi K, Ichikura T, et al. One-step nucleic acid amplification (OSNA) for the application of sentinel node concept in gastric cancer. Ann Surg Oncol. 2011;18:2289–96.
Kumagai K, Yamamoto N, Miyashiro I, Tomita Y, Katai H, Kushima R, et al. Multicenter study evaluating the clinical performance of the OSNA assay for the molecular detection of lymph node metastases in gastric cancer patients. Gastric Cancer. 2014;17:273–80.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma; 3rd English edition. Gastric Cancer. 2011;14:101–12.
UCC. TNM classification of malignant tumors. Weinheim: Wiley; 2009.
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification using loop primers. Mol Cell Probes. 2002;16:223–9.
Tsujimoto M, Nakabayashi K, Yoshiide K, Kaneko T, Iwase T, Akiyama F, et al. One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin Cancer Res. 2007;13:4807–16.
Tamaki Y. One-step nucleic acid amplification (OSNA): where do we go with it? Int J Clin Oncol. 2017;22:3–10.
Castellano I, Macri L, Deambroqio C, Balmativola D, Bussone R, Ala A, et al. Reliability of whole sentinel lymph node analysis by one-step nucleic acid amplification for intraoperative diagnosis of breast cancer metastases. Ann Surg. 2012;255:334–42.
Shoji Y, Kumagai K, Kamiya S, Ishida S, Nunobe S, Ohashi M, et al. Prospective feasibility study for single-tracer sentinel node mapping by ICG (indocyanine green) fluorescence and OSNA (one-step nucleic acid amplification) assay in laparoscopic gastric cancer surgery. Gastric Cancer. 2019;22:873–80.
Mcgrath S, Cross S, Pritchard SA. Histopathological assessment of lymph nodes in upper gastrointestinal cancer: does triple levelling detect significantly more metastases? J Clin Pathol. 2007;60:1222–5.
Mayanagi S, Takeuchi H, Kamiya S, Niihara M, Nakamura R, Takahashi T, et al. Suitability of sentinel node mapping as an index metastasis in early gastric cancer following endoscopic resection. Ann Surg Oncol. 2014;21:2987–93.
Nohara K, Goto O, Takeuchi H, Sasaki M, Maehata T, Yahagi N, et al. Gastric lymphatic flows may change before and after endoscopic submucosal dissection: in vivo porcine survival models. Gastric Cancer. 2019;22:723–30.
Acknowledgements
This study was supported by Sysmex Corporation and we would like to thank Sysmex Corp. for their enormous contribution to this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shimada, A., Takeuchi, H., Nishi, T. et al. Utility of the one-step nucleic acid amplification assay in sentinel node mapping for early gastric cancer patients. Gastric Cancer 23, 418–425 (2020). https://doi.org/10.1007/s10120-019-01016-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-019-01016-9