Abstract
Delayed perforation occurring after endoscopic submucosal dissection (ESD) is a rare but serious complication which sometimes requires emergent surgery. However, reports of its characteristics, including endoscopic imaging and management, are not fully detailed. A 70-year-old woman was referred to our hospital for the treatment of early gastric cancer. On the day of the ESD, hematemesis was observed because of a Mallory–Weiss tear, and a visible vessel in the post-ESD ulcer was additionally treated endoscopically by coagulation. Second-look endoscopic examination on the next day revealed a perforation 3 mm in diameter at the treated vessel in the ulcer. The shape of the perforation was round and the color of the surrounding muscle layer had become whitish. The perforation was closed with endoclips, and decompression of the pneumoperitoneum was performed. The patient was conservatively managed and was discharged 13 days after the ESD. We show endoscopic images of delayed perforation and discuss the mechanism and management of this complication.
Similar content being viewed by others
Introduction
Endoscopic submucosal dissection (ESD) achieves high curability by the en-bloc resection of a lesion, but the procedure carries a high risk of complications such as perforation. The risk of perforation during ESD is about 4% [1] and such a perforation can be conservatively treated by complete endoscopic closure [2]. In contrast, delayed perforation is a rare complication (0.1–0.45%) which occurs 1–2 days after the procedure, but often requires emergent surgery [3, 4]. Here, we report a patient with delayed perforation and provide endoscopic images of the perforation, as well as discussing the mechanism and management of this complication.
Case report
A 70-year-old woman was referred to our hospital for the treatment of a 30-mm early gastric cancer at the greater curvature of the antrum. ESD was performed with an insulated-tip (IT) knife (KD-610L; Olympus, Tokyo, Japan) using ICC-200 (Intelligent Cut and Coagulation; Erbe, Tübingen, Germany) as the electrosurgical unit; the procedure took 25 min. The resected specimen was 38 mm in size and pathological examination revealed a well-differentiated mucosal adenocarcinoma 26 mm in size. The created ulcer was carefully examined after the ESD, and visible vessels were coagulated with hot biopsy forceps (Radial Jaw 3; Boston Scientific, Natick, MA, USA) in the soft coagulation mode with 80-W current. Bleeding from a Mallory–Weiss tear occurred during ESD and ceased spontaneously without endoscopic hemostasis.
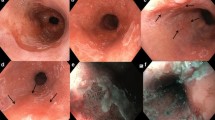
Six hours after the procedure, emergent endoscopic examination was performed because of hematemesis. The bleeding point was the Mallory–Weiss tear, which was coagulated using hot biopsy forceps. Additionally, a visible vessel in the post-ESD ulcer, which had been coagulated for several seconds during the ESD, was coagulated for about 1 s with hot biopsy forceps in the soft coagulation mode at 80 W, using ICC-200, in order to confirm whether or not the vessel was another bleeding point (Fig. 1).
On the next day, no physical findings or symptoms were present and a follow-up endoscopic examination was performed. The stomach did not expand well with air insufflation, and a perforation, 3 mm in diameter, was found in the post-ESD ulcer. The location of the perforation appeared to be coincident with that of the visible vessel coagulated during the ESD and emergent endoscopic examination. The shape of the perforation was round and the color of the surrounding muscle layer had become whitish, suggesting necrosis in a small part of the muscle layer. The perforation was closed with two endoclips (HX-600-090L; Olympus) (Fig. 2). During the endoscopic procedure, abdominal fullness due to air leakage from the perforated lesion was noted and free air was seen on the X-ray after endoscopy (Fig. 3). Decompression of the pneumoperitoneum was performed by using a 16G puncture needle with a side slit. A nasogastric tube was applied and suctioned intermittently. Management of the perforation consisted of 1 day of nasogastric suction, 5 days of fasting, and 7 days of intravenous antibiotic (cefazolin sodium) administration. The patient was discharged 13 days after the ESD.
Endoscopic examination 6 months after the ESD showed healing of the created ulcer.
Discussion
In the present report we show endoscopic images of delayed perforation occurring after an ESD. Whereas perforations during ESD are usually tear-like shape, the shape of the delayed perforation was round and the color of the surrounding muscle layer had become whitish, suggesting necrosis of the muscle layer related to the delayed perforation. The mechanism of delayed perforation is thought to be due to electrical cautery during submucosal dissection or repeated coagulation that causes ischemic change to the gastric wall resulting in necrosis [4]. But in our patient, shrinkage or disappearance of a vessel penetrating the gastric wall, caused by electrical cautery, might also have led to a small perforation, combined with necrosis of the surrounding muscle layer, because the size of the perforation was small and its location appeared to be coincident with that of the vessel coagulated during the ESD. Furthermore, in our patient, the delayed perforation was thought to have been caused mainly by coagulation during the ESD, although the additional short coagulation during the emergent endoscopic examination may have worsened the previous damage to the gastric wall and the visible vessel.
A summary of the clinicopathological features and clinical outcomes of 9 patients with delayed perforation after ESD (8 in previous reports [4–6] and our patient) is shown in Table 1. The mean time required for ESD in the five patients who received surgery was 3.6 h (range 1.5–9 h) and all of them had had sudden appearance of symptoms of peritoneal irritation. On the other hand, in our patient, the procedure time was 25 min and the perforation, 3 mm in diameter, was subclinical. Although the size of the perforation was not reported in most of the patients with surgery, except for case no. 1 (in whom the perforation was 20 mm in size) and case no. 8, as noted in Table 1, the mechanism of perforation could have been different in those patients and ours. The management of delayed perforation is surgical, if the perforation is found with peritonitis and the size is thought to be large [4], and there have been few reports of conservative treatment of delayed perforation [4–6]. Ours is the first case report in the English-language literature that shows the efficacy of endoscopic treatment with endoclips. In our patient, the necrosis of the surrounding muscle layer was limited and there was a viable muscle layer around the perforation.
So we were able to close the perforation with endoclips by stitching the viable muscle together. It was also fortunate that the perforation was diagnosed before the initiation of oral intake, because the management of peritonitis related to the leakage of gastric contents would be difficult without surgical treatment. In the case report of endoscopic closure in the Japanese literature [6], the diameter of the perforation was also small and endoscopic closure was performed on the day after the ESD. Thus, a small-size perforation and its early detection may be important for conservative treatment using endoclips.
In this report of delayed perforation after ESD, we showed endoscopic images of the delayed perforation and discussed the mechanism and management of this complication.
References
Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–42.
Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc. 2006;63:596–601.
Takizawa K, Hasuike N, Ikehara H, Inui T, Ono H. Management and prevention during endoscopic submucosal dissection (ESD). Endosc Dig. 2008;20:373–8. (in Japanese with an English abstract).
Hanaoka N, Uedo N, Ishihara R, Higashino K, Takeuchi Y, Inoue T, et al. Clinical features and outcomes of delayed perforation after endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2010;42:1112–5.
Onozato Y, Iizuka H, Sagawa T, Yoshimura S, Sakamoto I, Arai H, et al. A case report of delayed perforation dne to endoscopic submucosal dissection (ESD) for early gastric cancer. Progr Dig Endosc. 2006;68:114–5. (in Japanese).
Hirasawa T, Yamamoto Y, Okada K, Hayashi Y, Nego M, Kishihara T, et al. A case of the delayed perforation due to endoscopic submucosal dissection for the early gastric cancer of the residual stomach. Progr Dig Endosc. 2009;74:52–3. (in Japanese).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ikezawa, K., Michida, T., Iwahashi, K. et al. Delayed perforation occurring after endoscopic submucosal dissection for early gastric cancer. Gastric Cancer 15, 111–114 (2012). https://doi.org/10.1007/s10120-011-0089-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-011-0089-2