Abstract
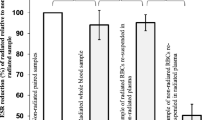
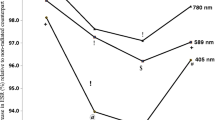
Low-level laser therapy (LLLT) is widely used in clinical practice for treatment of various pathologies. It is assumed that LLLT impact on microcirculation is among the mechanisms underlying its therapeutic effect. The microcirculation disorder is observed in the pathogenesis of any inflammatory process and is significantly influenced by red blood cells (RBCs). On this point, studying the RBCs morphology under the influence of LLLT on alterated organism is of scientific interest and practical importance. The aim of the present study was to analyze the LLLT effect on morphokinetic parameters of RBCs in hyperadrenalinemia. The LLLT effect was analyzed on rats intraperitoneally injected with adrenaline hydrochloride solution (0.1 mg/kg). As the comparison groups, the effects of LLLT, adrenaline, or saline injection as well as the parameters of intact animals were studied. LLLT was applied on the occipital region of rats for 10 min. The light irradiation with pulse frequency 415 Hz at 890 nm wavelength and average power density in the plane of the output window at 193 μW/cm2 was used. The dynamics of morphological characteristics of RBCs was studied by phase interference microscopy; the RBC electrophoretic mobility was tested by microelectrophoresis technique; photometric analyses of the RBCs amount, hemoglobin content, and osmotic fragility were performed. The adrenaline injection resulted in a significant increase in the amount of RBC pathological forms and a decrease in discocytes and normocytes by more than 50%. An increase in the optical density of RBC phase portraits, a decline in osmotic resistance, and electronegativity of RBC membranes and a reduction of their number in peripheral blood were also registered. The revealed effects persisted for 1 week after the adrenaline administration. LLLT did not significantly impact on the RBC parameters 1 h after adrenaline injection. However, a day later, LLLT reduced the severity of the adrenaline effect on RBSs, which was manifested in a decreased amount of the pathological forms of RBCs, restored RBC phase portraits, higher electrophoretic mobility and osmotic resistance, and RBSs amount in peripheral blood restored up to the level of intact animals. We suppose that the mechanism of LLLT action is realized both at cellular level through the laser radiation effect on RBC membranes, and at systemic level through the activation of stress-realizing systems of the organism with subsequent limitation of inflammatory response.







Similar content being viewed by others
References
Micheli L, Di Cesare Mannelli L, Lucarini E, Cialdai F, Vignali L, Ghelardini C, Monici M (2017) Photobiomodulation therapy by NIR laser in persistent pain: an analytical study in the rat. Lasers Med Sci. 32(8):1835–1846. https://doi.org/10.1007/s10103-017-2284-9
Ray M (2017) Laser therapy and osteoarthritis disability: an update of an unresolved topic. Nov Tech Arthritis Bone Res 2(2):555585. https://doi.org/10.19080/NTAB.2017.02.555585
Lio G-Y, Sun L, Liu TC-Y (2012) Aquaporin-1-mediated effects of low level He-Ne laser irradiation on human erythrocytes. Int J Photoenergy 2012:275209–275214. https://doi.org/10.1155/2012/275209
Joukar A, Nammakie E, Niroomand-Oscuii H (2015) A comparative study of thermal effects of 3 types of laser in eye: 3D simulation with bioheat equation. J Therm Biol 49–50:74–81. https://doi.org/10.1016/j.jtherbio.2015.02.004
Huang YY, Chen AC, Carroll JD, Hamblin MR (2011) Biphasic dose response in low level light therapy—an update. Formerly Nonlinear Biol Toxicol Med 9:602–618. https://doi.org/10.2203/dose-response.11-009.
Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI (2009) Absorption measurements of cell monolayers relevant to mechanisms of laser phototherapy: reduction or oxidation of cytochrome c oxidase under laser radiation at 632.8 nm. Photomed Laser Surg 26(6):593–599. https://doi.org/10.1089/pho.2008.2246
Barolet D, Duplay P, Jacomy H, Auclair M (2010) Importance of pulsing illumination parameters in low-level-light therapy. J Biomed Opt 15(4):048005–048007. https://doi.org/10.1117/1.3477186
Wu S, Xing D, Gao X, Chen WR (2009) High fluence lowpower laser irradiation induces mitochondrial permeability transition mediated by reactive oxygen species. J Cell Physiol 218:603–611. https://doi.org/10.1002/jcp.21636
Moriyama Y, Nguyen J, Akens M (2009) In vivo effects of low level laser therapy on inducible nitric oxide synthase. Lasers Surg Med 41(3):227–231. https://doi.org/10.1002/lsm.20745
Zhang L, Xing D, Zhu D, Chen Q (2008) Low-power laser irradiation inhibiting Abeta25-35-induced PC12 cell apoptosis via PKC activation. Cell Physiol Biochem 22(1–4):215–222. https://doi.org/10.1159/000149799
Liu H, Colavitti R, Rovira II, Finkel T (2005) Redox-dependent transcriptional regulation. Circ Res 97:967–974. https://doi.org/10.1161/01.RES.0000188210.72062.10
Rizzi CF, Mauriz JL, Freitas Corrкa DS et al (2006) Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg Med 38:704–713. https://doi.org/10.1002/lsm.20371
Aimbire F, Santos FV, Albertini R (2008) Low-level laser therapy decreases levels of lung neutrophils anti-apoptotic factors by a NF-kappaB dependent mechanism. Int Immunopharmacol 8(4):603–605. https://doi.org/10.1016/j.intimp.2007.12.007
Saygun I, Karacay S, Serdar M (2008) Effects of laser irradiation on the release of basic fibroblast growth factor (bFGF), insulin like growth factor-1 (IGF-1), and receptor of IGF-1 (IGFBP3) from gingival fibroblasts. Lasers Med Sci 23(2):211–215. https://doi.org/10.1007/s10103-007-0477-3
Kushibiki T, Hirasawa T, Okawa S, Ishihara M (2015) Low reactive level laser therapy for mesenchymal stromal cells therapies. Stem Cells Int 2015:974864, 12 page. https://doi.org/10.1155/2015/974864
Floras J (2009) Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol 54(5):375–385. https://doi.org/10.1371/journal.pone.0094234
Grassi G, Bolla G, Quarti-Trevano F, Arenare F, Brambilla G, Mancia G (2008) Sympathetic activation in congestive heart failure: reproducibility of neuroadrenergic markers. Eur J Heart Fail 10(12):1186–1191. https://doi.org/10.1016/j.ejheart.2008.09.013
Tashireva LA, Perelmuter VM, Denisov EV, Savelieva OE, Kaygorodova EV, Zavyalova MV, Manskikh VN (2017) Types of immune-inflammatory responses as a reflection of cell–cell interactions under conditions of tissue regeneration and tumor growth. Biochem (Moscow) 82(5):542–555. https://doi.org/10.1134/S0006297917050029
Chertok VM, Kotsyuba AE, Bespalova EV (2008) Role of nitric oxide in the reaction of arterial vessels to laser irradiation. Bull Exp Biol Med 145(6):751–754. https://doi.org/10.1007/s10517-008-0186-3
Hilário S, Saldanha C (2003) Martins e Silva J. An in vitro study of adrenaline effect on human erythrocyte properties in both gender. Clin Hemorheol Microcirc 28:89–98
Brazhe NA, Brazhe AR, Pavlov AN, Erokhova LA, Yusipovich AI, Maksimov GV, Mosekilde E, Sosnovtseva OV (2006) Unraveling cell processes: interference imaging interwoven with data analysis. J Biol Phys 32:191. https://doi.org/10.1007/s10867-006-9012-1
Boyarinov GA, Yakovleva EI, Zaitsev RR, Bugrova ML, Boyarinova LV, Solov’eva OD, Deryugina AV, Shumilova AV, Filippenko ES (2017) Pharmacological correction of microcirculation in rats suffering from traumatic brain injury. Cell Tissue Biol 11(1):65–72. https://doi.org/10.1134/S1990519X17010023
Moroz VV, Molchanova LV, Gerasimov LV, Ostapchenko DA, Yershova LI, Likhovetskaya ZM, Gorbunova NA (2006) Blood rheological and hemolytic effects of perfluorane in patients with severe injury and blood loss. Gen Reanimatol 2(1):5–11. https://doi.org/10.15360/1813-9779-2006-1-5-11
Kushibiki T, Hirasawa T, Okawa S, Ishihara M (2013) Blue laser irradiation generates intracellular reactive oxygen species in various types of cells. Photomed Laser Surg 31(3):95–104. https://doi.org/10.1089/pho.2012.3361
Forman HJ, Fukuto JM, Tottes M (2004) Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol 287(2):246–256. https://doi.org/10.1152/ajpcell.00516.2003
Xie Z (2003) Molecular mechanism of Na/K-ATPase-mediated signal transduction. Ann N Y Acad Sci USA 986:497–503. https://doi.org/10.1124/mi.3.3.157
Kucherenko YV, Wagner-Britz L, Bernhardt I, Lang F (2013) Effect of сhloride сhannel inhibitors on сytosolic Ca2+ levels and Ca2+-activated K+ (Gardos) сhannel activity in c human red blood cells. J Membrane Biol 246(4):315–326. https://doi.org/10.1007/s00232-013-9532-0
Andrews DA, Yang L, Low PS (2002) Phorbol ester stimulates s protein kinase C-mediated agtoxin-TK-sensitive calcium permeability pathway human red blood cell. Blood 100:3382–3392. https://doi.org/10.1182/blood.V100.9.3392
Maellaro E, Leoncini S, Moretti D et al (2013) Del Bello B., Tanganelli I., De Felice C., Ciccoli L. Erythrocyte caspase-3 activation and oxidative imbalance in erythrocytes and in plasma of type 2 diabetic patients. Acta Diabetol 50:489. https://doi.org/10.1007/s00592-011-0274-0
Koshkaryev A, Yedgar S, Relevy H (2003) Acridine orange induced translocation of phosphatidylserine of red blood cell surface. Am J Physiol Cell Physiol 285:720–722. https://doi.org/10.1152/ajpcell.00542.2002
Deryugina AV, Shumilova AV, Filippenko ES, Galkina YV, Simutis IS, Boyarinov GA (2017) Functional and biochemical parameters of erythrocytes during mexicor treatment in posttraumatic period after experimental blood loss and combined traumatic brain injury. Bull Exp Biol Med 164(1):26–29. https://doi.org/10.1007/s10517-017-3918-4
Sprague R, Ellsworth M, Stephenson A, Klein-henz M (2010) Deformation-induced ATP release from red blood cells requires CFTR activity. Am Physiol Soc 275:Н1726–Р1732
Yesman SS, Mamilov SO, Veligotsky DV, Gisbrecht AI (2016) Local changes in arterial oxygen saturation induced by visible and near-infrared light radiation. Lasers Med Sci 31(1):145–149. https://doi.org/10.1007/s10103-015-1838-y
Hamblin MR, Demidova-Rice TN (2007) Cellular chromophores and signaling in LLLT. In: Hamblin MR et al (eds) Mechanisms for low-light therapy II. The International Society for Optical Engineering, Bellingham. https://doi.org/10.1201/9781315364827
Chen AC, Arany PR, Huang YY, Tomkinson EM, Sharma SK et al (2011) Low level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLOS One. 6(7):e22453. https://doi.org/10.1371/journal.pone.0022453
Zhang J, Xing D, Gao X (2008) Low-power laser irradiation activates Src tyrosine through reactive oxygen species-mediated signaling pathway. J Cell Physiol 217(2):518–528. https://doi.org/10.1002/jcp.21697
Kujawa J, Pasternak-Mnich K, Zavodnik IB, Irzmański R (2014) The effect of near-infrared MLS laser radiation on cell membrane structure and radical generation. Lasers Med Sci 29(5):217. https://doi.org/10.1007/s10103-014-1571-y
Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM (2007) Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA 104(43):17063–17,068. https://doi.org/10.1073/pnas.0708160104
Deryugina AV, Ivashchenko MN, Ignatiev PS, Talamanova MN, Samodelkin AG (2018) The capabilities of interference microscopy in studying the in vitro state of erythrocytes exposed to low-intensity laser radiation for stress correction. Sovremennye Tehnol Med 10(4):78–83. https://doi.org/10.17691/stm2018.10.4.09
Deryugina AV, Ivashchenko MN, Ignatyev PS, Zolotova MV, Samodelkin АG (2018) The electrorophoretic mobility of erythrocytes and micronucleus test of buccal cells as a stress intensity marker. Int J Biomed 8(4):347–350. https://doi.org/10.21103/Article8(4)_OA16
Funding
The work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (contract no. 14.Z50.31.0022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animals were housed and the experiment was performed in accordance with Universal Declaration of animal rights and with the Order of the Ministry of Health of the Russian Federation No. 199n of April 1, 2016, “Approval of the good laboratory practice.” All procedures performed in studies involving animals were in accordance with the ethical standards of Lobachevsky University.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of Lobachevsky University.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deryugina, A.V., Ivashchenko, M.N., Ignatiev, P.S. et al. Low-level laser therapy as a modifier of erythrocytes morphokinetic parameters in hyperadrenalinemia. Lasers Med Sci 34, 1603–1612 (2019). https://doi.org/10.1007/s10103-019-02755-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02755-y