Abstract
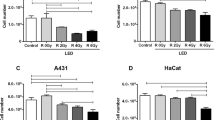
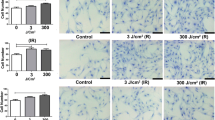
The treatment of squamous cell carcinoma (SCC) involves surgery, chemotherapy, and/or radiotherapy, which can cause mucositis (inflammation of the oral mucosa that causes considerable pain and can compromise the continuity of oncological treatment). Photobiomodulation (PBM) has been successfully used in the treatment of mucositis, but doubts arise regarding the use of laser for areas in which tumor cells may remain. In this study, the effect of PBM on the viability, mitochondrial activity, proliferation, apoptosis, and migration of cells derived from oral SCC was evaluated. SCC9 cells were irradiated with laser (660 and 780 nm, using 11 dosimetric parameters) and submitted to mitochondrial and caspase 3 activity tests after 1 and 3 days. Based on the results, cell viability (neutral red assay), proliferation (BrdU assay), and migration (scratch-wound assay) were evaluated using only the dosimetric parameters recommended for mucositis. Non-irradiated cells served as the control. The experiments were performed in triplicate. The 11 parameters diminished mitochondrial activity and induced tumor cell apoptosis. Using the parameters recommended for mucositis, irradiation with 780 nm (70 mW, 4 J/cm2) proved to be the safest and led to a reduction in cell viability, the induction of apoptosis, and a reduction in the migration capacity of the tumor cells.



Similar content being viewed by others
References
Marocchio LS, Lima J, Sperandio FF, Corrêa L, de Sousa SO (2010) Oral squamous cell carcinoma: an analysis of 1,564 cases showing advances in early detection. J Oral Sci 52(2):267–273
Sperandio FF, Giudice FS, Corrêa L, Pinto DS Jr, Hamblin MR, de Sousa SC (2013) Low-level laser therapy can produce increased aggressiveness of dysplastic and oral cancer cell lines by modulation of Akt/mTOR signaling pathway. J Biophotonics 6(10):839–847
Saba NF, Goodman M, Ward K, Flowers C, Ramalingam S, Owonikoko T, Chen A, Grist W, Wadsworth T, Beitler JJ, Khuri FR, Shin DM (2011) Gender and ethnic disparities in incidence and survival of squamous cell carcinoma of the oral tongue, base of tongue, and tonsils: a surveillance, epidemiology and end results program-based analysis. Oncology 81(1):12–20
Antunes HS, Ferreira EM, de Matos VD, Pinheiro CT, Ferreira CG (2008) The impact of low power laser in the treatment of conditioning-induced oral mucositis: a report of 11 clinical cases and their review. Med Oral Patol Oral Cir Bucal 13(3):E189–E192
Genot-Klastersky MT, Klastersky J, Awada F, Awada A, Crombez P, Martinez MD, Jaivenois MF, Delmelle M, Vogt G, Meuleman N, Paesmans M (2008) The use of low-energy laser (LEL) for the prevention of chemotherapy- and/or radiotherapy-induced oral mucositis in cancer patients: results from two prospective studies. Support Care Cancer 16(12):1381–1387
Simões A, Eduardo FP, Luiz AC, Campos L, Sá PH, Cristófaro M, Marques MM, Eduardo CP (2009) Laser phototherapy as topical prophylaxis against head and neck cancer radiotherapy-induced oral mucositis: comparison between low and high/low power lasers. Lasers Surg Med 41(4):264–270
Bensadoun RJ, Nair RG (2012) Low-level laser therapy in the prevention and treatment of cancer therapy-induced mucositis: 2012 state of the art based on literature review and meta-analysis. Curr Opin Oncol 24(4):363–370
Schartinger VH, Galvan O, Riechelmann H, Dudás J (2012) Differential responses of fibroblasts, non-neoplastic epithelial cells, and oral carcinoma cells to low-level laser therapy. Support Care Cancer 20(3):523–529
Zecha JA, Raber-Durlacher JE, Nair RG, Epstein JB, Sonis ST, Elad S, Hamblin MR, Barasch A, Migliorati CA, Milstein DM, Genot MT, Lansaat L, van der Brink R, Arnabat-Dominguez J, van der Molen L, Jacobi I, van Diessen J, de Lange J, Smeele LE, Schubert MM, Bensadoun RJ (2016) Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 1: mechanisms of action, dosimetric, and safety considerations. Support Care Cancer 24(6):2781–2792
Zecha JA, Raber-Durlacher JE, Nair RG, Epstein JB, Elad S, Hamblin MR, Barasch A, Migliorati CA, Milstein DM, Genot MT, Lansaat L, van der Brink R, Arnabat-Dominguez J, van der Molen L, Jacobi I, van Diessen J, de Lange J, Smeele LE, Schubert MM, Bensadoun RJ (2016) Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 2: proposed applications and treatment protocols. Support Care Cancer 24(6):2793–2805
Sonis ST, Hashemi S, Epstein JB, Nair RG, Raber-Durlacher JE (2016) Could the biological robustness of low level laser therapy (Photobiomodulation) impact its use in the management of mucositis in head and neck cancer patients. Oral Oncol 54:7–14
Russo G, Haddad R, Posner M, Machtay M (2008) Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist 13(8):886–898
Rosenthal DI, Trotti A (2009) Strategies for managing radiation-induced mucositis in head and neck cancer. Semin Radiat Oncol 19(1):29–34
Pinheiro AL, Carneiro NS, Vieira AL, Brugnera A Jr, Zanin FA, Barros RA, Silva PS (2002) Effects of low-level laser therapy on malignant cells: in vitro study. J Clin Laser Med Surg 20(1):23–26
Gomes Henriques AC, Ginani F, Oliveira RM, Keesen TS, Galvão Barboza CA, Oliveira Rocha HA, de Castro JF, Della Coletta R, de Almeida Freitas R (2014) Low-level laser therapy promotes proliferation and invasion of oral squamous cell carcinoma cells. Lasers Med Sci 29(4):1385–1395
Hawkins D, Houreld N, Abrahamse H (2005) Low level laser therapy (lllt) as an effective therapeutic modality for delayed wound healing. Ann N Y Acad Sci 1056:486–493
Myakishev-Rempel M, Stadler I, Brondon P, Axe DR, Friedman M, Nardia FB, Lanzafame R (2012) A preliminary study of the safety of red light phototherapy of tissues harboring cancer. Photomed Laser Surg 30(9):551–558
Gao X, Xing D (2009) Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci 16:4
Huang YY, Chen ACH, Carroll JD, Hamblin MR (2009) Biphasic dose response in low level light therapy. Dose Response 7:358–383
Huang YY, Sharma SK, Carroll J, Hamblin MR (2011) Biphasic dose response in low level light therapy - an update. Dose Response 9(4):602–618
Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR (2012) The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 40(2):516–533
AlGhamdi KM, Kumar A, Moussa NA (2012) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27(1):237–249
Dias Schalch T, Porta Santos Fernandes K, Costa-Rodrigues J, Pereira Garcia M, Agnelli Mesquita-Ferrari R, Kalil Bussadori S, Fernandes MH (2016) Photomodulation of the osteoclastogenic potential of oral squamous carcinoma cells. J Biophotonics 9(11–12):1136–1147
Nogueira GT, Mesquita-Ferrari RA, Souza NH, Artilheiro PP, Albertini R, Bussadori SK, Fernandes KP (2012) Effect of low-level laser therapy on proliferation, differentiation, and adhesion of steroid-treated osteoblastos. Lasers Med Sci 27:1189–1193
Fujihara NA, Hiraki KR, Marques MM (2006) Irradiation at 780 nm increases proliferation rate of osteoblasts independently of dexamethasone presence. Lasers Surg Med 38:332–336
Silva DF, Mesquita-Ferrari RA, Fernandes KP, Raele MP, Wetter NU, Deana AM (2012) Effective transmission of light for media culture, plates and tubes. Photochem Photobiol 88(5):1211–1216
Hughes SE (2003) Detection of apoptosis using in situ markers for DNA strand breaks in the failing human heart. Fact or epiphenomenon? J Pathol 201(2):181–186
Grivicich I, Regner A, da Rocha AB (2007) Morte Celular por Apoptose. Rev Bras Cancerol 53(3):335–343
Fotakis G, Timbrell JA (2006) In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett 160(2):171
Chiba K, Kawakami K, Tohyama K (1998) Simultaneous evaluation of cell viability by neutral red, MTT and crystal violet staining assays of the same cells. Toxicol in Vitro 12(3):251–258
Van Tonder A, Joubert AM, Cromarty AD (2015) Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res Notes 8:47
Mossmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Marshall NJ, Goodwin CJ, Holt SJ (1995) A critical assessment of the use of microculture tetrazolium assays to measure cell growth and function. Growth Regul 5(2):69–84
Hamblin MR (2017) Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys 4(3):337–361
Passarella S, Karu T (2014) Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol B 140:344–358
Wang S, Yu H, Wickliffe JK (2011) Limitation of the MTT and XTT assays for measuring cell viability due to superoxide formation induced by nano-scale TiO2. Toxicol in Vitro 25(8):2147–2151
Abrahamse H (2015) Stimulation of cellular proliferation and migration: is it a viable measure of photobiomodulation? Photomed Laser Surg 33(7):347–348
Morgan CD, Mills KC, Lefkowitz DL, Lefkowitz SS (1991) An improved colorimetric assay for tumor necrosis factor using WEHI 164 cells cultured on novel microtiter plates. J Immunol Methods 145(1–2):259–262
Iguchi H, Tanaka S, Ozawa Y, Kashiwakuma T, Kimura T, Hiraga T, Ozawa H, Kono A (1996) An experimental model of bone metastasis by human lung cancer cells: the role of parathyroid hormone-related protein in bone metastasis. Cancer Res 56:4040–4043
Deyama Y, Tei K, Yoshimura Y, Izumiyama Y, Takeyama S, Halta M, Totsuka Y, Suzuki K (2008) Oral squamous cell carcinomas stimulate osteoclast differentiation. Oncol Rep 20(3):663–668
Tang CH, Chuang JY, Fong YC, Maa MC, Way TD, Hung CH (2008) Bone-derived SDF-1 stimulates IL-6 release via CXCR4, ERK and NF-kappa B pathways and promotes osteoclastogenesis in human oral cancer cells. Carcinogenesis 29(8):1483–1492
Chuang FH, Hsue SS, Wu CW, Chen YK (2009) Immunohistochemical expression of RANKL, RANK, and OPG in human oral squamous cell carcinoma. J Oral Pathol Med 38(10):753–758
Tada T, Shin M, Fukushima H, Okabe K, Ozeki S, Okamoto M, Jimi E (2009) Oral squamous cell carcinoma cells modulate osteoclast function by RANKL-dependent and -independentmechanisms. Cancer Lett 274(1):126–131
Van Cann EM, Slootweg PJ, de Wilde PC, Otte-Höller I, Koole R, Stoelinga PJ, Merkx MA (2009) The prediction of mandibular invasion by squamous cell carcinomas with the expression of osteoclast-related cytokines in biopsy specimens. Int J Oral Maxillofac Surg 38(3):279–284
Jimi E, Furuta H, Matsuo K, Tominaga K, Takahashi T, Nakanishi O (2011) The cellular and molecular mechanisms of bone invasion by oral squamous cell carcinoma. Oral Dis 17(5):462–468
Honig A, Rieger L, Kapp M, Krockenberger M, Eck M, Dietl J, Kammerer U (2006) Increased tartrate resistant acid phosphatase (TRAP) expression in malignant breast, ovarian and melanoma tissue: an investigational study. BMC Cancer 6:199
How J, Brown JR, Saylor S, Rimm DL (2014) Macrophage expression of tartrate-resistant acid phosphatase as a prognostic indicator in colon cancer. Histochem Cell Biol 142(2):195–204
Vladimirov YA, Klebanov GI, Borisenko GG, Osipov AN (2004) Molecular and cellular mechanisms triggered by low-level laser irradiation. Biophysics 49:325–336
Kreslavski VD, Fomina IR, Los DA, Carpentier R, Kuznetsov VV, Allakhverdiev SI (2012) Red and near infra-red signaling: hypothesis and perspectives. J Photochem Photobiol C: Photochem Rev 13(3):190–203
Bailes HJ, Lucas RJ (2013) Human melanopsin forms a pigment maximally sensitive to blue light (lambdamax approximately 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades. Proc Biol Sci 280:20122987
Hamblin MR (2018) Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol 94(2):199–212
Fekrazad R, Chiniforush N (2014) Oral mucositis prevention and management by therapeutic laser in head and neck cancers. J Lasers Med Sci 5(1):1–7
Funding
This study was supported by São Paulo Research Foundation (grant no. 2013/07502-1). KPSF, SKB, and RAMF were supported by CNPq-National Council for Technological and Scientific Development (CNPq, grant nos. 311078/2015-0, 305905/2014-7, and 305739/2014-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Schalch, T.D., Fernandes, M.H., Destro Rodrigues, M.F.S. et al. Photobiomodulation is associated with a decrease in cell viability and migration in oral squamous cell carcinoma. Lasers Med Sci 34, 629–636 (2019). https://doi.org/10.1007/s10103-018-2640-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2640-4