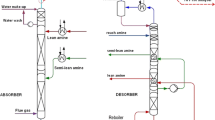
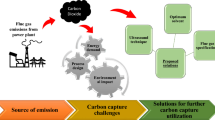
Abstract
Carbon dioxide (CO2), one of the green house gases (GHGs) is well known for more than a century. Its emission from the combustion of fossil fuels in addition to other industrial sources is adversely affecting the climate on earth. Climate change is emerging as a risk all over the world that has generated public concern. Estimates have indicated that power production contributes to the tune of 70% of the total CO2 released into the atmosphere from fossil fuel combustion worldwide. Capturing and securely storing CO2 from the global combustion systems thus constitutes an important and achievable target. A legion of researchers have thus far developed absorbents to remove CO2 from combustion facilities that are currently recognized globally as most effective. The cost of capturing CO2 can be reduced by finding a low-cost solvent that can minimize energy requirements, equipment size, and corrosion. Monoethanolamine is being used for removing CO2 from the exhaust streams and is a subject inculcated over a period of about last 80 odd years. Host of such other amines are being investigated and put into practice. However, commercializations of such operating plants for capturing CO2 from power plants in the world are few and far between. On the other hand, aqueous ammonia is the other chemical solvent for capturing CO2 that has proven experimentally to be more effective than amine-based processes. This communication aims at critically elucidating relative merits and demerits of ammonia and amine-based CO2 capture options from the exhausts of coal fired thermal power plants (TPPs). It includes the life cycle CO2 emissions for both the processes. Finally, it is estimated that a total emission of about 152 Mt CO2-equivalent could occur after use of 100 Mt ammonium bicarbonate (NH4HCO3) as synthetic N-fertilizer that is about 50% of the total CO2 captured (315 Mt) for producing the fertilizer, NH4HCO3. Clearly, this estimate demonstrates that the synthetic N-fertilizer, NH4HCO3, produced by NH3 scrubbing of CO2 from fossil fuel (e.g., coal) fired TPP could have a significant beneficial environmental impact so far as GHG emission is concerned.
Similar content being viewed by others
References
Ali SH (2005) Kinetics of the reaction of carbon dioxide with blends of amines in aqueous media using the stopped-flow technique. Int J Chem Kinet 37:391–405
Ali SH, Merchant SQ, Fahim MA (2002) Reaction kinetics of some secondary alkanolamines with carbon dioxide in aqueous solutions by stopped flow technique. Sep Purif Technol 27:121–136
Aronua UE, Svendsena HF, Hoffb KA, Juliussenb O (2009) Solvent selection for carbon dioxide absorption. Energy Procedia 1:1051–1057
Astarita G (1967) Mass transfer with chemical reaction. Elsevier, Amsterdam
Bai H, Wei JH (1996) The CO2 mitigation options for the electric sector: a case study of Taiwan. Energy Policy 24:221–228
Bai H, Yeh AC (1997) Removal of CO2 greenhouse gas by ammonia scrubbing. Ind Eng Chem Res 36(6):2490–2493
Banks FE (2000) The Kyoto negotiations on climate change—an economic perspective. Energy Sources 22:481–496
Blok K, Worrell E, Caelenaere R, Turkenburg W (1993) The cost effectiveness of CO2 emission reduction achieved by energy conservation. Energy Policy 25:656–667
Blomen E, Hendriks C, Neele F (2009) Capture technologies: improvements and promising developments. Energy Procedia 1:1505–1512
Bolin B (1998) The Kyoto negotiations on climate change: a science perspective. Science 279:330–331
Bottoms RR (1930) U.S. process for separating acid gases. U.S. Patent 1,783,901
Brooks R (1953) Manufacture of ammonium bicarbonate. British Patent 742,386
Brooks LA, Audrieth LF (1946) Ammonium carbamate. Inorg Synth 2:85–86
Cansolv (2009) Breakthrough CO2 capture technology. Cansolv Technologies, Inc. http://www.cansolv.com/. Retrieved October 9, 2009
Chakma A (1995) Separation of CO2 and SO2 from flue gas streams by liquid membranes. Energy Convers Manag 36:405–410
Chakravarti S, Gupta A, Hunek B (2001) Advanced technology for the capture of carbon dioxide from flue gases. In: Proceedings of the 1st national conference on carbon sequestration, Washington, DC, May 15–17
Cheng Z, Ma Y, Li X, Zhang WPZ (2007) Investigation of carbon distribution with 14C as tracer for carbon dioxide (CO2) sequestration through NH4HCO3 production. Energy Fuels 21(6):3334–3340
Chludzinski GR, Stogryn EL, Weichert S (1986) Commercial experience with Flexsorb SE absorbent. Presented at the 1986 Spring AIChE national meeting, New Orleans, LA
Choi W-J, Min B-M, Shon B-H, Seo J-B, Oh K-J (2009a) Characteristics of absorption/regeneration of CO2–SO2 binary systems into aqueous AMP + ammonia solutions. J Ind Eng Chem 15:635–640
Choi W-J, Min B-M, Seo J-B, Park S-W, Oh K-J (2009b) Effect of ammonia on the absorption kinetics of carbon dioxide into aqueous 2-amino-2-methyl-1-propanol solutions. Ind Eng Chem Res 48:4022–4029
Ciferno JP, DiPietro P, Tarka T (2005) An economic scoping study for CO2 capture using aqueous ammonia. DOE/NETL final report, revised, February
Corti A, Lombardi L (2004) Reduction of carbon dioxide emissions from a SCGT/CC by ammonia solution absorption—preliminary results. Int J Thermodyn 7(4):173–181
Danckwerts PV (1970) Gas–liquid reactions. McGraw-Hill, New York
Danckwerts PV, Sharma MM (1966) The absorption of carbon dioxide into solutions of alkalies and amines. Chem Eng 44:244–280
Darde V, Thomsen K, van Well WJM, Stenby EH (2009) Chilled ammonia process for CO2 capture. Energy Procedia 1:1035–1042
Dave N, Do T, Puxty G, Rowland R, Feron PHM, Attalla MI (2009) CO2 capture by aqueous amines and aqueous ammonia—a comparison. Energy Procedia 1:949–954
Derks PWJ, Versteeg GF (2009) Kinetics of absorption of carbon dioxide in aqueous ammonia solutions. Energy Procedia 1:1139–1146
Diao YF, Zheng XY, He BS, Chen CH, Xu XC (2004) Experimental study on capturing CO2 greenhouse gas by ammonia scrubbing. Energy Convers Manag 45:2283–2296
Dooley JJ, Davidson CL, Dahowski RT (2009) An assessment of the commercial availability of carbon dioxide capture and storage technologies as of June 2009. Prepared for the U.S. Department of Energy under Contract DE-AC05-76RL01830. Pacific Northwest National Laboratory, Richland
Gal E (2006) Ultra cleaning combustion gas including the removal of CO2. World Intellectual Property, Patent WO 2006022885
Goldstein AM (1983) Commercialization of a new gas treating agent. Presented at petro-energy ‘83 conference, Houston, TX, September 14
Hakka LE, Ouimet MA (2006) Method for recovery of CO2 from gas streams. US Patent and Trademark Office Granted Patent. Cansolv Technologies Inc., Assignee. US Patent 7056482 B2. June 6
Harald W, Randall S, Lwazikazi T (2002) Comparing developing countries under potential carbon allocation schemes. Clim Policy 2:303–318
Hatch TF, Pigford RL (1962) Simultaneous absorption of carbon dioxide and ammonia in water. Ind Eng Chem Fundam 1:209–214
He Q, Chen M, Meng L, Liu K, Pan W-P (2010) Study on carbon dioxide removal from flue gas by absorption of aqueous ammonia. http://www.netl.doe.gov/publications/proceedings/04/carbon-seq/158.pdf. Accessed March 18, 2010
Huang JP (1993) Energy substitution to reduce carbon dioxide emission in China. Energy 18:281–287
IEA (2007) IEA greenhouse gas R&D programme (IEA GHG). CO2 Capture Ready Plants, 2007/4, May
IPCC (1990) Policymaker’s summary of the scientific assessment of climate change. Report to IPCC from working group. Meteorological Office, Branknell
IPCC (1997) Revised 1996 IPCC guidelines for national greenhouse gas inventories. Meteorological Office, Branknell
IPCC (2005) Special report on carbon dioxide capture and storage. Prepared by working group III of the intergovernmental panel on climate change, Cambridge University Press, New York, USA
Kaplan LJ (1982) Cost-saving process recovers CO2 from power-plant flue gas. Chem Eng 89(24):30–31
Kim JY, Han K, Chun HD (2009) CO2 absorption with low concentration ammonia liquor. Energy Procedia 1:757–762
Kimura N, Omata K, Kiga T, Takano S, Shikisma S (1995) Characteristics of pulverized coal combustion in O2–CO2 mixtures for CO2 recovery. Energy Convers Manag 36:805–808
Kohl AL, Nielsen RB (1997) Gas purification, 5th edn. Gulf Publishing Company Book Division, Texas, chap 2, pp 40–186
Koornneef J, von Keulen T, Faajj A, Turkenburg W (2008) Life cycle assessment of a pulverized coal power plant with post-combustion capture, transport and storage of CO2. Int J Greenh Gas Control 2:448–467
Koutinas AA, Yianoulis P, Lycourghiods A (1983) Industrial scale modelling of the thermochemical energy storage system based on CO2 + 2NH3 ↔ NH2COONH4 equilibrium. Energy Convers Manag 23:55–61
Kozak F, Petig A, Morris E, Rhudy R, Thimsen D (2009) Chilled ammonia process for CO2 capture. Energy Procedia 1:1419–1426
Lee JW, Li R (2003) Integration of fossil energy systems with CO2 sequestration through NH4HCO3 production. Energy Convers Manag 44:1535–1546
Lee D-H, Cho W-J, Moon S-J, Ha S-H, Kim I-G, Oh K-J (2008) Characteristics of absorption and regeneration of carbon dioxide in aqueous 2-amino-2-methyl-1-propanol/ammonia solutions. Korean J Chem Eng 25(2):279–284
Li M-H, Chang B-C (1995) Solubility of mixtures of carbon dioxide and hydrogen sulfide in water + monoethanolamine + 2-amino-2-methyl-1-propanol. J Chem Eng Data 40(1):328–331
Liu J, Wang S, Zhao B, Tong H, Chen C (2009) Absorption of carbon dioxide in aqueous ammonia. Energy Procedia 1:933–940
Lu YY, Huang Y, Zhang W, Zheng XH (2007) Estimation of chemical fertilizer N-induced direct N2O emission from China agricultural fields in 1991–2000 based on GIS technology. Yingyong Shengtai Xuebao 18:1539–1545 (in Chinese)
Lynn S, Straatemeier JR, Kramers H (1955) Absorption studies in the light of penetration theory—I. Long wetted-wall columns. Chem Eng Sci 4:49–57
Martin MH, Meyer S (1999) Greenhouse gas carbon dioxide mitigation. Lewis Publishers, Boca Raton
McLarnon CR, Duncan JL (2009) Testing of ammonia based CO2 capture with multi-pollutant control technology. Energy Procedia 1:1027–1034
Mshewa MM, Rochelle GT (1994) Carbon dioxide absorption/desorption kinetics in blended amines. In: Proceedings of 44th annual Laurance Reid gas conditioning conference, University of Oklahoma, Norman, OK, February 27–March 2, p 251
Nishikawa N, Hiroano A, Ikuta Y (1995) Photosynthetic efficiency improvement by microalgae cultivation in tubular type reactor. Energy Convers Manag 36:681–684
Nsakala N, Marion J, Bozzuto C, Liljedahl G, Palkes M, Vogel D, Gupta JC, Guha M, Johnson H, Plasynski S (2001) Engineering feasibility of CO2 capture on an existing US coal fired power plant. In: Proceedings of the 1st national conference on carbon sequestration, Washington, DC, May 15–17
Paul S, Ghoshal AK, Mandal B (2007) Removal of CO2 by single and blended aqueous alkanolamine solvents in hollow-fiber membrane contactor: modeling and simulation. Ind Eng Chem Res 46:2576–2588
Pauley CR, Simiskey PL, Haigh S (1984) N-Ren recovers CO2 from flue gas economically. Oil Gas J 82(20):87–92
Plummer LN, Parkhurst DL, Thorstenson DC (1983) Development of reaction models for ground-water systems. Geochim Cosmochim Acta 47:665–686
Qi NZ, Cheng GY, Yi LW (2010) Experimental studies on removal of carbon dioxide by aqueous ammonia fine spray. Sci China Technol Sci 53(1):117–122
Rao AB (2002) Details of a technical, economic and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control; Appendix to Annual technical progress report [DE-FC26-00NT40935]. Prepared for U.S. Department of Energy, National Energy Technology Laboratory, Morgantown, West Virginia, USA
Resnik KP, Yeh JT, Pennline HW (2004) Aqua ammonia process for the simultaneous removal of CO2, SO2 and NO x . Int J Environ Technol Manag 4(1/2):89–104
Sartori G, Savage DW (1983) Sterically hindered amines for carbon dioxide removal from gases. Ind Eng Chem Fundam 22:239–249
Sass BM (2002) Purity specifications for commodity uses of carbon dioxide in the United States. Battelle. October 25
Seo DJ, Hong WH (2000) Effect of piperazine on the kinetics of carbon dioxide with aqueous solutions of 2-amino-2-methyl-1-propanol. Ind Eng Chem Res 39:2062–2067
Shale CC, Simpson DG, Lewis PS (1971) Removal of sulfur and nitrogen oxides from stack gases by ammonia. Chem Eng Prog Symp Ser 67:52–57
Sun JW (2002) The Kyoto negotiations on climate change—an arithmetic perspective. Energy Policy 30:83–85
Sun JW (2003) The natural and social properties of CO2 emission intensity. Energy Policy 31:203–209
UNFCCC (1997) Kyoto protocol to the United Nations Framework Convention on Climate Change (UNFCCC). UNFCCC/CP/1997/L.7/Add.1, Bonn
Valenti G, Bonalumi D, Macchi E (2009) Energy and exergy analyses for the carbon capture with the chilled ammonia process (CAP). Energy Procedia 1:1059–1066
Wang S, Liu F, Chen C, Xu X (2007) Life cycle emissions of greenhouse gas for ammonia scrubbing technology. Korean J Chem Eng 24(3):495–498
Wolsky AM, Daniels EJ, Jody BJ (1994) CO2 capture from the flue gas of conventional fossil-fuel-fired power plants. Environ Prog 13:214–219
Xi Z, Shi X, Liu M, Cao Y, Wu X, Ru G (1985) Agrochemical properties of ammonium bicarbonate. Turang Xuebao 22(3):223–232
Yan XY, Akiyama H, Yagi K, Akimoto H (2009) Global estimations of the inventory and mitigation potential of methane emissions from rice cultivation conducted using the 2006 intergovernmental panel on climate change guidelines. Glob Biogeochem Cycle 23:GB2002. doi:10.1029/2008GB003299
Yeh AC, Bai H (1999) Comparison of ammonia and monoethanolamine solvents to reduce CO2 greenhouse gas emissions. Sci Total Environ 228:121–133
You JK, Park H, Yang SH, Hong WH, Shin W, Kang JK, Yi KB, Kim J (2008) Influence of additives including amine and hydroxyl groups on aqueous ammonia absorbent for CO2 capture. J Phys Chem B 112(14):4323–4328
Zhou J, Shang J, Der V, Li Z, Zhang J, Li X, Zhang Z (2009) A feasibility study on a two stage benefits CO2 sequestration technology for fossil fuel power generation. www.netl.doe.gov/publications/proceedings/01/carbon_seq/p2.pdf. Accessed June 14, 2009
Zucong C (2009) Contribution of N-fertilization to N2O emissions from crop lands of China and mitigation options. In: 7th IFA annual conference, Shanghai, China, May 25–27
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bandyopadhyay, A. Amine versus ammonia absorption of CO2 as a measure of reducing GHG emission: a critical analysis. Clean Techn Environ Policy 13, 269–294 (2011). https://doi.org/10.1007/s10098-010-0299-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-010-0299-z