Abstract
Purpose
This study aimed to evaluate and compare the presence of genes related to surface proteins between isolates of Streptococcus pneumoniae from healthy carriers (HC) and invasive pneumococcal disease (IPD) with a particular focus on serotype 19A.
Methods
The presence of these genes was identified by real-time PCR. Subsequently, we employed the Galleria mellonella larval infection model to study their effect on pathogenicity in vivo.
Results
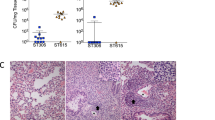
The percentage of selected virulence genes was similar between the HC and IPD groups (p > 0.05), and the genes lytA, nanB, pavA, pcpA, phtA, phtB, phtE, rrgA, and sipA were all present in both groups. However, the virulence profile of the isolates differed individually between HC and IPD groups. The highest lethality in G. mellonella was for IPD isolates (p < 0.01), even when the virulence profile was the same as compared to the HC isolates or when the nanA, pspA, pspA-fam1, and pspC genes were not present.
Conclusions
The occurrence of the investigated virulence genes was similar between HC and IPD S. pneumoniae serotype 19A groups. However, the IPD isolates showed a higher lethality in the alternative G. mellonella model than the HC isolates, regardless of the virulence gene composition, indicating that other virulence factors may play a decisive role in virulence. Currently, this is the first report using the in vivo G. mellonella model to study the virulence of clinical isolates of S. pneumoniae.


Similar content being viewed by others
Data availability
All relevant data are available in the manuscript.
Code availability
Not applicable.
References
Centers for Disease Control and Prevention (CDC) (2021) Global pneumococcal disease and vaccine. Last updated: 27 January 2022. Available online: https://www.cdc.gov/pneumococcal/global.html. Accessed 4 Mar 2022
World Health Organization (2018) Vaccine-preventable diseases surveillance standards. Influenza Last Updated: 5 September 2018. Available online: https://www.who.int/publications/i/item/surveillance-standards-for-vaccine-preventable-diseases-2nd-edition. Accessed 4 Mar 2022
Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SR, Reiner RC (2018) Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 18(11):1191–1210. https://doi.org/10.1016/S1473-3099(18)30310-4
BOOK, CDC Pink (2015) Pneumococcal disease. Chapter, v. 15, p 217–229. Available online: https://www.cdc.gov/vaccines/pubs/pinkbook/pneumo.html. Accessed 4 Mar 2022
Keshava S, Magisetty J, Tucker TA, Kujur W, Mulik S, Esmon CT, aL, (2021) Endothelial cell protein C receptor deficiency attenuates streptococcus pneumoniae–induced pleural fibrosis. Am J Respir Cell Mol Biol 1(64):477–491. https://doi.org/10.1165/rcmb.2020-0328OC
Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C et al (2015) Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev 28:871–899. https://doi.org/10.1128/CMR.00024-15
Ganaie F, Saad JS, Mcgee L, van Tonder AJ, Bentley SD, Lo SW et al (2020) A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral Streptococcus. MBio. https://doi.org/10.1128/mBio10.1128/mBio
Mott MP, Caierão J, Cunha GR, del Maschi MM, Pizzutti K, d’Azevedo P et al (2019) Emergence of serotype 19A Streptococcus pneumoniae after PCV10 associated with a ST320 in adult population, in Porto Alegre, Brazil. Epidemiol Infect 147. https://doi.org/10.1017/S0950268819000013
Yahiaoui RY, Bootsma HJ, den Heijer CDJ, Pluister GN, John Paget W, Spreeuwenberg P et al (2018) Distribution of serotypes and patterns of antimicrobial resistance among commensal Streptococcus pneumoniae in nine European countries. BMC Infect Dis 29:18. https://doi.org/10.1186/s12879-018-3341-0
Masomian M, Ahmad Z, Gew LT, Poh CL (2020) Development of next generation streptococcus pneumoniae vaccines conferring broad protection. Vaccines (Basel) 1:8. https://doi.org/10.3390/vaccines8010132
Brandileone MCC, Almeida SCG, Minamisava R, Andrade AL (2018) Distribution of invasive Streptococcus pneumoniae serotypes before and 5 years after the introduction of 10-valent pneumococcal conjugate vaccine in Brazil. Vaccine 3(36):2559–2566. https://doi.org/10.1016/j.vaccine.2018.04.010
Isturiz R, Sings HL, Hilton B, Arguedas A, Reinert RR, Jodar L (2017) Streptococcus pneumoniae serotype 19A: worldwide epidemiology. Expert Rev Vaccines 3(16):1007–1027. https://doi.org/10.1080/14760584.2017.1362339
Chamkhaleh MA, Esteghamati A, Sayyahfar S, Gandomi-Mohammadabadi A, Balasi J, Abdiaei H et al (2020) Serotype distribution of Streptococcus pneumoniae among healthy carriers and clinical patients: a systematic review from Iran. Eur J Clin Microbiol Infect Dis 1(39):2257–2267. https://doi.org/10.1007/s10096-020-03963-z
Mitchell AM, Mitchell TJ (2010) Streptococcus pneumoniae: virulence factors and variation. Clin Microbiol Infect 16:411–418. https://doi.org/10.1111/j.1469-0691.2010.03183.x
Galán-Bartual S, Pérez-Dorado I, García P, Hermoso JA (2015) Structure and function of choline-binding proteins. In: Streptococcus pneumoniae: molecular mechanisms of host-pathogen interactions. Elsevier Inc., pp 207–230. https://doi.org/10.1016/B978-0-12-410530-0.00011-9
Morais V, Texeira E, Suarez N (2019) Next-generation whole-cell pneumococcal vaccine Vaccines (Basel) 1:7. https://doi.org/10.3390/vaccines7040151
Corsini B, Aguinagalde L, Ruiz S, Domenech M, Yuste J (2021) Vaccination with lyta, lytc, or pce of streptococcus pneumoniae protects against sepsis by inducing iggs that activate the complement system. Vaccines (Basel) 9:1–15. https://doi.org/10.3390/vaccines9020186
Kadioglu A, Weiser JN, Paton JC (2008) Andrew PW (2008) The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol 6:288–301. https://doi.org/10.1038/nrmicro1871
UniProt (2021) Uniprot [Internet]. Retrieved 2 February 2023, from https://www.uniprot.org/uniprotkb?query=streptococcus%20pneumoniae
Reglinski M, Ercoli G, Plumptre C, Kay E, Petersen FC, Paton JC et al (2018) A recombinant conjugated pneumococcal vaccine that protects against murine infections with a similar efficacy to Prevnar-13. npj Vaccines 1:3. https://doi.org/10.1038/s41541-018-0090-4
Janapatla RP, Chen CL, Hsu MH, Liao WT, Chiu CH (2018) Immunization with pneumococcal neuraminidases NanA, NanB and NanC to generate neutralizing antibodies and to increase survival in mice. J Med Microbiol 1(67):709–723. https://doi.org/10.1099/jmm.0.000724
André GO, Assoni L, Rodriguez D, Leite LCC, dos Santos TEP, Ferraz LFC et al (2020) Immunization with PhtD truncated fragments reduces nasopharyngeal colonization by Streptococcus pneumoniae. Vaccine 27(38):4146–4153. https://doi.org/10.1016/j.vaccine.2020.04.050
Zhang Y, Masi AW, Barniak V, Mountzouros K, Hostetter MK, Green BA (2001) Recombinant PhpA protein, a unique histidine motif-containing protein from Streptococcus pneumoniae, protects mice against intranasal pneumococcal challenge. Infect Immun 69:3827–3836. https://doi.org/10.1128/IAI.69.6.3827-3836.2001
Anderson RJ, Guru S, Weeratna R, Makinen S, Falconer DJ, Sheppard NC et al (2016) In vivo screen of genetically conserved Streptococcus pneumoniae proteins for protective immunogenicity. Vaccine 7(34):6292–6300. https://doi.org/10.1016/j.vaccine.2016.10.061
Nakahashi-Ouchida R, Uchida Y, Yuki Y, Katakai Y, Yamanoue T, Ogawa H et al (2021) A nanogel-based trivalent PspA nasal vaccine protects macaques from intratracheal challenge with pneumococci. Vaccine 8(39):3353–64. https://doi.org/10.1016/j.vaccine.2021.04.069
Visan L, Rouleau N, Proust E, Peyrot L, Donadieu A, Ochs M (2018) Antibodies to PcpA and PhtD protect mice against Streptococcus pneumoniae by a macrophage- and complement-dependent mechanism. Hum Vaccin Immunother 1(14):489–494. https://doi.org/10.1080/21645515.2017.1403698
Moschioni M, Emolo C, Biagini M, Maccari S, Pansegrau W, Donati C et al (2010) The two variants of the Streptococcus pneumoniae pilus 1 RrgA adhesin retain the same function and elicit cross-protection in vivo. Infect Immun 78:5033–5042. https://doi.org/10.1128/IAI.00601-10
Prieto P, Burton J, Graepel R, Price A, Whelan M, Worth A (2014) EURL ECVAM strategy to replace, reduce and refine the use of animals in the assessment of acute mammalian systemic toxicity. Publications Office of the European Union, Luxembourg
Doke SK, Dhawale SC (2015) Alternatives to animal testing: a review. Saudi Pharmaceutical Journal 23(3):223–229
Singkum P, Suwanmanee S, Pumeesat LN (2019) A powerful in vivo alternative model in scientific research: Galleria mellonella. Acta Microbiol Immunol Hung. https://doi.org/10.1556/030.66.2019.001
Ramarao N, Nielsen-Leroux C, Lereclus D (2012) The insect Galleria mellonella as a powerful infection model to investigate bacterial pathogenesis. J Vis Exp. https://doi.org/10.3791/4392
Vilcinskas A (2011) Insects emerge as valuable model hosts to explore virulence. Virulence 2:376–378. https://doi.org/10.4161/viru.2.5.18289
Pinto HB, Brust FR, Macedo AJ, Trentin DS (2020) The antivirulence compound myricetin possesses remarkable synergistic effect with antibacterials upon multidrug resistant Staphylococcus aureus. Microb Pathog 1:149. https://doi.org/10.1016/j.micpath.2020.104571
Luther MK, Arvanitis M, Mylonakis E, LaPlante KL (2014) Activity of daptomycin or linezolid in combination with rifampin or gentamicin against biofilm-forming Enterococcus faecalis or E. faecium in an in vitro pharmacodynamic model using simulated endocardial vegetations and an in vivo survival assay using Galleria mellonella larvae. Antimicrob Agents Chemother 58:4612–4620. https://doi.org/10.1128/AAC.02790-13
Sheehan G, Dixon A, Kavanagh K (2019) Utilization of galleria mellonella larvae to characterize the development of staphylococcus aureus infection. Microbiology (United Kingdom) 165:863–875. https://doi.org/10.1099/mic.0.000813
Silva LN, Campos-Silva R, Ramos LS, Trentin DS, Macedo AJ, Branquinha MH et al (2018) Virulence of Candida haemulonii complex in Galleria mellonella and efficacy of classical antifungal drugs: a comparative study with other clinically relevant non-albicans Candida species. FEMS Yeast Res 1:18. https://doi.org/10.1093/femsyr/foy082
Campos-Silva R, Brust FR, Trentin DS, Macedo AJ (2019) Alternative method in Galleria mellonella larvae to study biofilm infection and treatment. Microb Pathog 1:137. https://doi.org/10.1016/j.micpath.2019.103756
Kaito C, Murakami K, Imai L, Furuta K (2020) Animal infection models using non-mammals. Microbiol Immunol 1(64):585–592. https://doi.org/10.1111/1348-0421.12834
Glavis-Bloom J, Muhammed M, Mylonakis E (2012) Of model hosts and man: Using Caenorhabditis elegans, Drosophila melanogaster and Galleria mellonella as model hosts for infectious disease research. Adv Exp Med Biol 710:11–17. https://doi.org/10.1007/978-1-4419-5638-5_2
Cools F, Torfs E, Aizawa J, Vanhoutte B, Maes L, Caljon G et al (2019) Optimization and characterization of a Galleria mellonella larval infection model for virulence studies and the evaluation of therapeutics against Streptococcus pneumoniae. Front Microbiol 10:311. https://doi.org/10.3389/fmicb.2019.00311
Evans BA, Rozen DE (2012) A Streptococcus pneumoniae infection model in larvae of the wax moth Galleria mellonella. Eur J Clin Microbiol Infect Dis 31:2653–2660. https://doi.org/10.1007/s10096-012-1609-7
Bárria C, Mil-Homens D, Pinto SN, Fialho AM, Arraiano CM, Domingues S (2022) RNase R, a new virulence determinant of Streptococcus pneumoniae. Microorganisms 10(2):317. https://doi.org/10.3390/microorganisms10020317
Gazioglu O, Kareem BO, Afzal M, Shafeeq S, Kuipers OP, Ulijasz AT, Andrew PW, Yesilkaya H (2021) Glutamate dehydrogenase (GdhA) of Streptococcus pneumoniae is required for high temperature adaptation. Infect Immun. 89(12):0040021. https://doi.org/10.1128/IAI.00400-21
Cools F, Triki D, Geerts N, Delputte P, Fourche Cos D (2020) In vitro and in vivo evaluation of in silico predicted pneumococcal UDPG: PP inhibitors. Front Microbiol 11:1596. https://doi.org/10.3389/fmicb.2020.01596
Blumental S, Granger-Farbos A, Moïsi JC, Soullié B, Leroy P, Njanpop-Lafourcade BM et al (2015) Virulence factors of Streptococcus pneumonia. Comparison between African and French invasive isolates and implication for future vaccines. PLoS ONE 27:10. https://doi.org/10.1371/journal.pone.0133885
Weiser JN, Ferreira DM, Paton JC (2018) Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol 1(16):355–367. https://doi.org/10.1038/s41579-018-0001-8
Mills RO, Abdullah MR, Akwetey SA, Sappor DC, Cole I, Baffuor-Asare M et al (2020) Post-vaccination Streptococcus pneumoniae carriage and virulence gene distribution among children less than five years of age, cape coast. Ghana Microorganisms 1(8):1–18. https://doi.org/10.3390/microorganisms8121987
Kawaguchiya M, Urushibara N, Aung MS, Shinagawa M, Takahashi S, Kobayashi N (2019) Prevalence of various vaccine candidate proteins in clinical isolates of streptococcus pneumoniae: characterization of the novel pht fusion proteins phta/b and phta/d. Pathogens 1:8. https://doi.org/10.3390/pathogens8040162
Zhao W, Pan F, Wang B, Wang C, Sun Y, Zhang T et al (2019) Epidemiology characteristics of Streptococcus pneumoniae from children with pneumonia in shanghai: a retrospective study. Front Cell Infect Microbiol 9:258. https://doi.org/10.3389/fcimb.2019.00258
Pettigrew MM, Fennie KP, York MP, Daniels J, Ghaffar F (2006) Variation in the presence of neuraminidase genes among Streptococcus pneumoniae isolates with identical sequence types. Infect Immun 74:3360–3365. https://doi.org/10.1128/IAI.01442-05
Imai S, Ito Y, Ishida T, Hirai T, Ito I, Yoshimura K et al (2011) Distribution and clonal relationship of cell surface virulence genes among Streptococcus pneumoniae isolates in Japan. Clin Microbiol Infect 17:1409–1414. https://doi.org/10.1111/j.1469-0691.2010.03446.x
Yan Z, Cui Y, Zhou W, Li W, Tan X, Chen W et al (2019) Molecular characterization of Streptococcus pneumoniae in children living in southwest China and assessment of a potential protein vaccine, rPfbA. Vaccine 29(37):721–731. https://doi.org/10.1016/j.vaccine.2018.12.021
Brooks WA, Chang LJ, Sheng X, Hopfer R, de Bruyn G, Bologa M et al (2015) Safety and immunogenicity of a trivalent recombinant PcpA, PhtD, and PlyD1 pneumococcal protein vaccine in adults, toddlers, and infants: A phase I randomized controlled study. Vaccine 26(33):4610–4617. https://doi.org/10.1016/j.vaccine.2015.06.078
Khan MN, Pichichero ME (2012) Vaccine candidates PhtD and PhtE of Streptococcus pneumoniae are adhesins that elicit functional antibodies in humans. Vaccine 30(18):2900–2907. https://doi.org/10.1016/j.vaccine.2012.02.023
Cornick JE, Tastan Bishop Ö, Yalcin F, Kiran AM, Kumwenda B, Chaguza C et al (2017) The global distribution and diversity of protein vaccine candidate antigens in the highly virulent Streptococcus pnuemoniae serotype 1. Vaccine 7(35):972–980. https://doi.org/10.1016/j.vaccine.2016.12.037
Žemličková H, Mališová L, Španělová P, Jakubů V, Kozáková J, Musílek M et al (2018) Molecular characterization of serogroup 19 Streptococcus pneumoniae in the Czech Republic in the post-vaccine era. J Med Microbiol 1(67):1003–1011. https://doi.org/10.1099/jmm.0.000765
Gholamhosseini-Moghaddam T, Rad M, Mousavi SF, Ghazvini K (2015) Detection of lytA, pspC, and rrgA genes in Streptococcus pneumoniae isolated from healthy children. Iran J Microbiol 7(3):156–160
Pinto TC, Costa NS, Pina SE, Souza AR, Oliveira LM, Moura CA, Teixeira LM (2020) Virulence-associated characteristics of serotype 14 and serogroup 9 Streptococcus pneumoniae clones circulating in Brazil: association of penicillin non-susceptibility with transparent colony phenotype variants. Front Microbiol 11:2009
Knupp-Pereira PA, Torres N, Marques C, Lúcia & , Teixeira M, Cardoso H, et al (2020) Prevalence of PspA families and pilus islets among Streptococcus pneumoniae colonizing children before and after universal use of pneumococcal conjugate vaccines in Brazil. Brazilian Journal of Microbiology [Internet] 51:419–425. https://doi.org/10.1007/s42770-019-00179-y10.1007/s42770-019-00179-y/Published
Velikova N, Kavanagh K, Wells JM (2016) Evaluation of Galleria mellonella larvae for studying the virulence of Streptococcus suis. BMC Microbiol 15(16):1–9. https://doi.org/10.1186/s12866-016-0905-2
Six A, Krajangwong S, Crumlish M, Zadoks RN, Walker D (2019) Galleria mellonella as an infection model for the multi-host pathogen Streptococcus agalactiae reflects hypervirulence of strains associated with human invasive disease. Virulence 1(10):600–609. https://doi.org/10.1080/21505594.2019.1631660
Subramanian K, Henriques-Normark B, Normark S (2019) Emerging concepts in the pathogenesis of the Streptococcus pneumoniae: from nasopharyngeal colonizer to intracellular pathogen. Cell Microbiol 1:21. https://doi.org/10.1111/cmi.13077
Tsai CJ-Y, Loh JMS, Proft T (2016) Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 7(3):214–229. https://doi.org/10.1080/21505594.2015.1135289
Selva L, Ciruela P, Blanchette K, del Amo E, Pallares R, Orihuela CJ, Muñoz-Almagro C (2012) Prevalence and clonal distribution of pcpA, psrP and Pilus-1 among pediatric isolates of Streptococcus pneumoniae. PLoS One 7(7):e41587. https://doi.org/10.1371/journal.pone.0041587
Žemličková H, Mališová L, Španělová P, Jakubů V, Kozáková J, Musílek M, Medvecký M (2018) Molecular characterization of serogroup 19 Streptococcus pneumoniae in the Czech Republic in the post-vaccine era. J Med Microbiol 67(7):1003. https://doi.org/10.1099/jmm.0.000765
Acknowledgements
We thankfully acknowledge Nicole Hiller Bondarczuk for the technical assistance in G. mellonella laboratory housing, Grasiela Agnes for helping us with the amplicon sequencing, and the UFCSPA Research Support Nucleus, especially Cristiane Bündchen from Núcleo de Apoio à Pesquisa e Pós-Graduação (Nupesq) – PROPPG by the statistical assistance.
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil—Universal grant number 443150/2014–1 and fellowships—process number 88887.597507/2021–00 and grant number 308617/2021–5) and by the Fundação de Apoio à Pesquisa do Estado do Rio Grande do Sul (FAPERGS/Brazil—PRONEM process number 1871–25511/13–4 and Public Notice FAPERGS/MS/CNPq/SESRS n. 03/2017—PPSUS—grant agreement 17/2551–0001453-7) and also to UFCSPA (Programa de fomento a realização de projetos de pesquisa na UFCSPA—07/2020/PROPPG).
Author information
Authors and Affiliations
Contributions
Josiane Trevisol Leal: conceptualization, methodology, validation, formal analysis, investigation, writing—original draft, and writing—review and editing. Amanda de Carvalho Robaina: investigation and visualization. Kauana Pizzutti: investigation, resources, and visualization. Mariana Preussler Mott: investigation, resources, and visualization. Muriel Primon-Barros: conceptualization, methodology, validation, formal analysis, resources, writing—review and editing, supervision, project administration, and funding acquisition. Danielle Silva Trentin: conceptualization, methodology, validation, formal analysis, resources, writing—review and editing, supervision, project administration, and funding acquisition. Cícero Armídio Gomes Dias: conceptualization, methodology, validation, formal analysis, resources, writing—review and editing, supervision, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Federal University of Health Sciences of Porto Alegre. Institutional Ethics Committee: 2.176.785 and 1.115.418.
Consent to participate
Informed consent was obtained from all individual participants included in the study or their parents or legal guardian in the case of children under 16.
Consent for publication
The authors affirm that human research participants provided informed consent for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leal, J.T., Primon-Barros, M., de Carvalho Robaina, A. et al. Streptococcus pneumoniae serotype 19A from carriers and invasive disease: virulence gene profile and pathogenicity in a Galleria mellonella model. Eur J Clin Microbiol Infect Dis 42, 399–411 (2023). https://doi.org/10.1007/s10096-023-04560-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04560-6