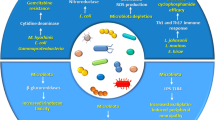
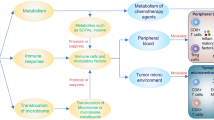
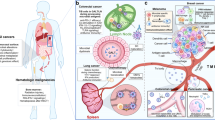
Abstract
Cancer being an increasing burden on human health, the use of anticancer drugs has risen over the last decades. The physiological effects of these drugs are not only perceived by the host’s cells but also by the microbial cells it harbors as commensals, notably the gut microbiota. Since the early ‘50 s, the cytotoxicity of anticancer chemotherapy was evaluated on bacteria revealing some antimicrobial activities that result in an established perturbation of the gut microbiota. This perturbation can affect the host’s health through dysbiosis, which can lead to multiple complications, but has also been shown to have a direct effect on the treatment efficiency.
We, therefore, conducted a review of literature focusing on this triangular relationship involving the microbial communities from the gut, the host’s disease, and the anticancer treatment. We focused specifically on the antimicrobial effects of anticancer chemotherapy, their impact on mutagenesis in bacteria, and the perspectives of using bacteria-based tools to help in the diagnostic and treatment of cancer.


Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Melichar B, Dvorák J, Hyspler R, Zadák Z (2005) Intestinal permeability in the assessment of intestinal toxicity of cytotoxic agents. Chemotherapy 51:336–338. https://doi.org/10.1159/000088957
Pusztaszeri MP, Genta RM, Cryer BL (2007) Drug-induced injury in the gastrointestinal tract: clinical and pathologic considerations. Nat Clin Pract Gastroenterol Hepatol 4:442–453. https://doi.org/10.1038/ncpgasthep0896
Montassier E, Al-Ghalith GA, Ward T, Corvec S, Gastinne T, Potel G et al (2016) Pretreatment gut microbiome predicts chemotherapy-related bloodstream infection. Genome Med 28(8):49. https://doi.org/10.1186/s13073-016-0301-4
Samet A, Śledzińska A, Krawczyk B, Hellmann A, Nowicki S, Kur J et al (2013) Leukemia and risk of recurrent Escherichia coli bacteremia: genotyping implicates E. coli translocation from the colon to the bloodstream. Eur J Clin Microbiol Infect Dis 32:1393–400. https://doi.org/10.1007/s10096-013-1886-9
Mikulska M, Del Bono V, Bruzzi P, Raiola AM, Gualandi F, Van Lint MT et al (2012) Mortality after bloodstream infections in allogeneic haematopoietic stem cell transplant (HSCT) recipients. Infection 40:271–278. https://doi.org/10.1007/s15010-011-0229-y
Hobson CA, Bonacorsi S, Fahd M, Baruchel A, Cointe A, Poey N et al (2019) Successful treatment of bacteremia due to NDM-1-producing Morganella morganii with aztreonam and ceftazidime-avibactam combination in a pediatric patient with hematologic malignancy. Antimicrob. Agents Chemother 63. https://doi.org/10.1128/AAC.02463-18
Hobson CA, Bonacorsi S, Hocquet D, Baruchel A, Fahd M, Storme T et al (2020) Impact of anticancer chemotherapy on the extension of beta-lactamase spectrum: an example with KPC-type carbapenemase activity towards ceftazidime-avibactam. Sci Rep 17(10):1–8. https://doi.org/10.1038/s41598-020-57505-w
Hobson CA, Bonacorsi S, Jacquier H, Choudhury A, Magnan M, Cointe A et al (2020) KPC beta-lactamases are permissive to insertions and deletions conferring substrate spectrum modifications and resistance to ceftazidime-avibactam. Antimicrob. Agents Chemother [cited 2020 11];64. https://aac.asm.org/content/64/12/e01175-20. https://doi.org/10.1128/AAC.01175-20
Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A et al (2012) Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 1(55):905–914. https://doi.org/10.1093/cid/cis580
Montassier E, Gastinne T, Vangay P, Al-Ghalith GA, Bruley des Varannes S, Massart S et al (2015) Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol Ther. 42:515–28. https://doi.org/10.1111/apt.13302
Hess AS, Kleinberg M, Sorkin JD, Netzer G, Johnson JK, Shardell M et al (2014) Prior colonization is associated with increased risk of antibiotic-resistant Gram-negative bacteremia in cancer patients. Diagn Microbiol Infect Dis 79:73–76. https://doi.org/10.1016/j.diagmicrobio.2014.01.022
Haeusler GM, Mechinaud F, Daley AJ, Starr M, Shann F, Connell TG et al (2013) Antibiotic-resistant Gram-negative bacteremia in pediatric oncology patients–risk factors and outcomes. Pediatr Infect Dis J 32:723–726. https://doi.org/10.1097/INF.0b013e31828aebc8
Perez F, Adachi J, Bonomo RA (2014) Antibiotic-resistant Gram-negative bacterial infections in patients with cancer. Clin Infect Dis Off Publ Infect Dis Soc Am 15(59):S335–S339. https://doi.org/10.1093/cid/ciu612
Mikulska M, Viscoli C, Orasch C, Livermore DM, Averbuch D, Cordonnier C et al (2014) Aetiology and resistance in bacteraemias among adult and paediatric haematology and cancer patients. J Infect 68:321–331. https://doi.org/10.1016/j.jinf.2013.12.006
Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C et al (2017) Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol Off J Eur Soc Med Oncol 1(28):1368–1379. https://doi.org/10.1093/annonc/mdx108
Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L et al (2016) Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 2:7. https://doi.org/10.1038/ncomms10391
Aarnoutse R, Ziemons J, Penders J, Rensen SS, de Vos-Geelen J, Smidt ML (2019 ) The clinical link between human intestinal microbiota and systemic cancer therapy. Int J Mol Sci [cited 2019 30];20. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6747354/. https://doi.org/10.3390/ijms20174145
Schroeder BO, Bäckhed F (2016) Signals from the gut microbiota to distant organs in physiology and disease. Nat Med 6(22):1079–1089. https://doi.org/10.1038/nm.4185
Jia W, Li H, Zhao L, Nicholson JK (2008) Gut microbiota: a potential new territory for drug targeting. Nat Rev Drug Discov 7:123–129. https://doi.org/10.1038/nrd2505
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM et al (2015) Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 27(350):1084–1089. https://doi.org/10.1126/science.aac4255
Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D et al (2013) The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 22(342):971–976. https://doi.org/10.1126/science.1240537
van Vliet MJ, Tissing WJE, Dun CAJ, Meessen NEL, Kamps WA, de Bont ESJM et al (2009) Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis Off Publ Infect Dis Soc Am 15(49):262–270. https://doi.org/10.1086/599346
Pope JL, Tomkovich S, Yang Y, Jobin C (2017) Microbiota as a mediator of cancer progression and therapy. Transl Res J Lab Clin Med 179:139–154. https://doi.org/10.1016/j.trsl.2016.07.021
Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM (2017) Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol 14:356–365. https://doi.org/10.1038/nrgastro.2017.20
Severyn CJ, Brewster R, Andermann TM (2019) Microbiota modification in hematology: still at the bench or ready for the bedside? Blood Adv 12(3):3461–3472. https://doi.org/10.1182/bloodadvances.2019000365
Ichim TE, Kesari S, Shafer K (2018) Protection from chemotherapy- and antibiotic-mediated dysbiosis of the gut microbiota by a probiotic with digestive enzymes supplement. Oncotarget 20(9):30919–30935. https://doi.org/10.18632/oncotarget.25778
Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. Int J Cancer 15(118):3030–3044. https://doi.org/10.1002/ijc.21731
Bossuet-Greif N, Vignard J, Taieb F, Mirey G, Dubois D, Petit C et al (2018) The colibactin genotoxin generates DNA interstrand cross-links in infected cells. mBio [cited 2021 6];9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5874909/. https://doi.org/10.1128/mBio.02393-17
Zou S, Fang L, Lee M-H (2018) Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol Rep 6:1–12. https://doi.org/10.1093/gastro/gox031
Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan T-J et al (2012) Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 5(338):120–123. https://doi.org/10.1126/science.1224820
Dziubańska-Kusibab PJ, Berger H, Battistini F, Bouwman BAM, Iftekhar A, Katainen R et al (2020) Colibactin DNA-damage signature indicates mutational impact in colorectal cancer. Nat Med 26:1063–1069. https://doi.org/10.1038/s41591-020-0908-2
Pleguezuelos-Manzano C, Puschhof J, Rosendahl Huber A, van Hoeck A, Wood HM, Nomburg J et al (2020) Mutational signature in colorectal cancer caused by genotoxic pks + E. coli. Nature 580:269–73. https://doi.org/10.1038/s41586-020-2080-8
Cougnoux A, Delmas J, Gibold L, Faïs T, Romagnoli C, Robin F et al (2016) Small-molecule inhibitors prevent the genotoxic and protumoural effects induced by colibactin-producing bacteria. Gut 1(65):278–285. https://doi.org/10.1136/gutjnl-2014-307241
Bruneau A, Baylatry M-T, Joly AC, Sokol H (2018) Gut microbiota: what impact on colorectal carcinogenesis and treatment? Bull Cancer (Paris) 105:70–80. https://doi.org/10.1016/j.bulcan.2017.10.025
Chang AH, Parsonnet J (2010) Role of bacteria in oncogenesis. Clin Microbiol Rev 23:837–857. https://doi.org/10.1128/CMR.00012-10
Lamm DL (2000) Efficacy and safety of Bacille Calmette-Guérin immunotherapy in superficial bladder cancer. Clin Infect Dis 1(31):S86-90. https://doi.org/10.1086/314064
Redelman-Sidi G, Glickman MS, Bochner BH (2014) The mechanism of action of BCG therapy for bladder cancer—a current perspective. Nat Rev Urol 11:153–162. https://doi.org/10.1038/nrurol.2014.15
Chen J, Zhao K-N, Vitetta L (2019) Effects of intestinal microbial−elaborated butyrate on oncogenic signaling pathways. Nutrients 7:11. https://doi.org/10.3390/nu11051026
Basson A, Trotter A, Rodriguez-Palacios A, Cominelli F (2016) Mucosal interactions between genetics, diet, and microbiome in inflammatory bowel disease. Front Immunol [cited 2020 21];7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4970383/. https://doi.org/10.3389/fimmu.2016.00290
Sivaprakasam S, Prasad PD, Singh N (2016) Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol Ther 1(164):144–151. https://doi.org/10.1016/j.pharmthera.2016.04.007
Garrett WS (2015) Cancer and the microbiota. Science 3(348):80–86. https://doi.org/10.1126/science.aaa4972
Mager D (2006) Bacteria and cancer: cause, coincidence or cure? A review. J Transl Med 28(4):14. https://doi.org/10.1186/1479-5876-4-14
Lopez-Siles M, Duncan SH, Garcia-Gil LJ, Martinez-Medina M (2017) Faecalibacterium prausnitzii : from microbiology to diagnostics and prognostics. ISME J 11:841–852. https://doi.org/10.1038/ismej.2016.176
Duncan SH, Louis P, Flint HJ (2004) Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol 70:5810–5817. https://doi.org/10.1128/AEM.70.10.5810-5817.2004
Ye H, Adane B, Khan N, Alexeev E, Nusbacher N, Minhajuddin M et al (2018) Subversion of systemic glucose metabolism as a mechanism to support the growth of leukemia cells. Cancer Cell 8(34):659-673.e6. https://doi.org/10.1016/j.ccell.2018.08.016
Flores GE, Caporaso JG, Henley JB, Rideout JR, Domogala D, Chase J et al (2014) Temporal variability is a personalized feature of the human microbiome. Genome Biol [cited 2020 20];15. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4252997/.https://doi.org/10.1186/s13059-014-0531-y
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W et al (2012) Host-gut microbiota metabolic interactions. Science 8(336):1262–1267. https://doi.org/10.1126/science.1223813
Mandal RS, Saha S, Das S (2015) Metagenomic surveys of gut microbiota. Genomics Proteomics Bioinformatics 13:148–158. https://doi.org/10.1016/j.gpb.2015.02.005
Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A et al (2019) What is the healthy gut microbiota composition? A Changing ecosystem across age, environment, diet, and diseases. microorganisms [cited 2020 2];7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6351938/. https://doi.org/10.3390/microorganisms7010014
Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett C, Knight R, Gordon JI (2007) The human microbiome project: exploring the microbial part of ourselves in a changing world. Nature 18(449):804–810. https://doi.org/10.1038/nature06244
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C et al (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65. https://doi.org/10.1038/nature08821
Kho ZY, Lal SK (2018) The human gut microbiome – a potential controller of wellness and disease. Front Microbiol [cited 2020 9];9. https://www.frontiersin.org/articles/10.3389/fmicb.2018.01835/. https://doi.org/10.3389/fmicb.2018.01835
Ratajczak W, Rył A, Mizerski A, Walczakiewicz K, Sipak O, Laszczyńska M (2019) Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim Pol 4(66):1–12. https://doi.org/10.18388/abp.2018_2648
Rooks MG, Garrett WS (2016) Gut microbiota, metabolites and host immunity. Nat Rev Immunol 27(16):341–352. https://doi.org/10.1038/nri.2016.42
Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI (2005) Host-bacterial mutualism in the human intestine. Science 25(307):1915–1920. https://doi.org/10.1126/science.1104816
Bilotta AJ, Ma C, Yang W, Yu Y, Yu Y, Zhao X et al (2021) Propionate enhances cell speed and persistence to promote intestinal epithelial turnover and repair. Cell Mol Gastroenterol Hepatol 1(11):1023–1044. https://doi.org/10.1016/j.jcmgh.2020.11.011
Morrison DJ, Preston T (2016) Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 3(7):189–200. https://doi.org/10.1080/19490976.2015.1134082
Chattopadhyay I, Nandi D, Nag A (2020) The pint- sized powerhouse: illuminating the mighty role of the gut microbiome in improving the outcome of anti- cancer therapy. Semin. Cancer Biol [cited 2020 10];https://www.sciencedirect.com/science/article/pii/S1044579X20301693. https://doi.org/10.1016/j.semcancer.2020.07.012
Goodman B, Gardner H (2018) The microbiome and cancer. J Pathol 244:667–676. https://doi.org/10.1002/path.5047
Lopetuso LR, Petito V, Graziani C, Schiavoni E, Sterbini FP, Poscia A et al (2018) Gut microbiota in health, diverticular disease, irritable bowel syndrome, and inflammatory bowel diseases: time for microbial marker of gastrointestinal disorders. Dig Dis 36:56–65. https://doi.org/10.1159/000477205
De Angelis M, Francavilla R, Piccolo M, De Giacomo A, Gobbetti M (2015) Autism spectrum disorders and intestinal microbiota. Gut Microbes 6:207–213. https://doi.org/10.1080/19490976.2015.1035855
Cox AJ, West NP, Cripps AW (2015) Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol 3:207–215. https://doi.org/10.1016/S2213-8587(14)70134-2
Rajagopala SV, Yooseph S, Harkins DM, Moncera KJ, Zabokrtsky KB, Torralba MG et al (2016) Gastrointestinal microbial populations can distinguish pediatric and adolescent acute lymphoblastic leukemia (ALL) at the time of disease diagnosis. BMC Genomics [cited 2021 1];17. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4986186/. https://doi.org/10.1186/s12864-016-2965-y
Bai L, Zhou P, Li D, Ju X (2017) Changes in the gastrointestinal microbiota of children with acute lymphoblastic leukaemia and its association with antibiotics in the short term. J Med Microbiol 66:1297–1307. https://doi.org/10.1099/jmm.0.000568
Vicente-Dueñas C, Janssen S, Oldenburg M, Auer F, González-Herrero I, Casado-García A et al (2020) An intact gut microbiome protects genetically predisposed mice against leukemia. Blood 29(136):2003–2017. https://doi.org/10.1182/blood.2019004381
Burns MB, Lynch J, Starr TK, Knights D, Blekhman R (2015) Virulence genes are a signature of the microbiome in the colorectal tumor microenvironment. Genome Med 24(7):55. https://doi.org/10.1186/s13073-015-0177-8
Yu J, Feng Q, Wong SH, Zhang D, Liang Qy, Qin Y et al (2017) Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 66:70–8. https://doi.org/10.1136/gutjnl-2015-309800
Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H et al (2019) Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 1(68):1014–1023. https://doi.org/10.1136/gutjnl-2017-315084
Ohigashi S, Sudo K, Kobayashi D, Takahashi O, Takahashi T, Asahara T et al (2013) Changes of the intestinal microbiota, short chain fatty acids, and fecal pH in patients with colorectal cancer. Dig Dis Sci 1(58):1717–1726. https://doi.org/10.1007/s10620-012-2526-4
Alhinai EA, Walton GE, Commane DM (2019) The role of the gut microbiota in colorectal cancer causation. Int J Mol Sci 24(20):5295. https://doi.org/10.3390/ijms20215295
Vivarelli S, Salemi R, Candido S, Falzone L, Santagati M, Stefani S et al (2019) Gut microbiota and cancer: from pathogenesis to therapy. Cancers 11:38. https://doi.org/10.3390/cancers11010038
Cheng WY, Wu C-Y, Yu J (2020) The role of gut microbiota in cancer treatment: friend or foe? Gut 1(69):1867–1876. https://doi.org/10.1136/gutjnl-2020-321153
Arruebo M, Vilaboa N, Sáez-Gutierrez B, Lambea J, Tres A, Valladares M et al (2011) Assessment of the evolution of cancer treatment therapies. Cancers 3:3279–3330. https://doi.org/10.3390/cancers3033279
Montassier E, Batard E, Massart S, Gastinne T, Carton T, Caillon J et al (2014) 16S rRNA gene pyrosequencing reveals shift in patient faecal microbiota during high-dose chemotherapy as conditioning regimen for bone marrow transplantation. Microb Ecol 67:690–699. https://doi.org/10.1007/s00248-013-0355-4
Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA et al (2013) Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 22(342):967–970. https://doi.org/10.1126/science.1240527
Guðmundsdóttir JS, Fredheim EGA, Koumans CIM, Hegstad J, Tang P-C, Andersson DI et al (2021) The chemotherapeutic drug methotrexate selects for antibiotic resistance. EBioMedicine [cited 2021 15];74. https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(21)00536-3/. https://doi.org/10.1016/j.ebiom.2021.103742
Nakamura S, Oda Y, Shimada T, Oki I, Sugimoto K (1987) SOS-inducing activity of chemical carcinogens and mutagens in Salmonella typhimurium TA1535/pSK1002: examination with 151 chemicals. Mutat Res Lett 1(192):239–246. https://doi.org/10.1016/0165-7992(87)90063-7
Meunier A, Nerich V, Fagnoni-Legat C, Richard M, Mazel D, Adotevi O et al (2019) Enhanced emergence of antibiotic-resistant pathogenic bacteria after in vitro induction with cancer chemotherapy drugs. J Antimicrob Chemother 1(74):1572–1577. https://doi.org/10.1093/jac/dkz070
Foley GE, McCarthy RE, Binns VM, Snell EE, Guirard BM, Kidder GW et al (1958) A comparative study of the use of microorganisms in the screening of potential antitumor agents*. Ann N Y Acad Sci 76:413–441. https://doi.org/10.1111/j.1749-6632.1958.tb54862.x
Schabel FM, Pittillo RF (1961) Screening for and biological characterization of antitumor agents using microorganisms11the work reported here that was conducted in the authors’ laboratories was supported by Contract No. SA-43-ph-1809 with the Cancer Chemotherapy National Service Center, National Cancer Institute, National Institutes of Health. In: Umbreit WW, editor. Advances in Applied Microbiology. Academic Press; [cited 2022 7]. p. 223–56.https://www.sciencedirect.com/science/article/pii/S0065216408705112. https://doi.org/10.1016/S0065-2164(08)70511-2
Falzone L, Salomone S, Libra M (2018) Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol [cited 2022 12];9. https://www.frontiersin.org/article/10.3389/fphar.2018.01300
Hoff DDV, Slavik M, Muggia F(2020) 5-Azacytidine. Ann Intern Med [cited 2021 14]. https://www.acpjournals.org/doi/abs/10.7326/0003-4819-85-2-237
Bischoff KB, Dedrick RL, Zaharko DS, Longstreth JA (1971) Methotrexate pharmacokinetics. J Pharm Sci 60:1128–1133. https://doi.org/10.1002/jps.2600600803
Teller MN (1961) Division of microbiology: antibiotics in experimental cancer chemotherapy*. Trans N Y Acad Sci 24:158–166. https://doi.org/10.1111/j.2164-0947.1961.tb00759.x
Paces V, Doskocil J, Sorm F (1968) The effect of 5-azacytidine on the synthesis of ribosomes in escherichia coli. FEBS Lett 1:55–58. https://doi.org/10.1016/0014-5793(68)80017-1
Bodet CA, Jorgensen JH, Drutz DJ (1985) Antibacterial activities of antineoplastic agents. Antimicrob Agents Chemother 28:437–439
Michel J, Jacobs JY, Sacks T (1979) Bactericidal effect of combinations of antimicrobial drugs and antineoplastic antibiotics against gram-negative bacilli. Antimicrob Agents Chemother 16:761–766
Levy HB (1963) Effect of actinomycin d on hela cell nuclear RNA metabolism. Proc Soc Exp Biol Med Soc Exp Biol Med N Y N 113:886–9. https://doi.org/10.3181/00379727-113-28521
Hamilton-Miller JM (1984) Antimicrobial activity of 21 anti-neoplastic agents. Br J Cancer 49:367–369
Benedict WF, Baker MS, Haroun L, Choi E, Ames BN (1977) Mutagenicity of cancer chemotherapeutic agents in the Salmonella/microsome test. Cancer Res 37:2209–2213
Beale G (1993) The discovery of mustard gas mutagenesis by Auerbach and Robson in 1941. Genetics 1(134):393–399
Iyer VN, Szybalski W (1958) Two simple methods for the detection of chemical mutagens. Appl Microbiol 6:23–29
Scherr GH, Fishman M, Weaver RH (1954) The mutagenicity of some carcinogenic compounds for Escherichia Coli. Genetics 39:141–149
Ames BN, Lee FD, Durston WE (1973) An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci U S A 70:782–786
Ames BN (1973) Carcinogens are mutagens: their detection and classification. Environ Health Perspect 6:115–118
Mortelmans K, Zeiger E (2000) The Ames Salmonella/microsome mutagenicity assay. Mutat Res 20(455):29–60
Dunkel VC, Zeiger E, Brusick D, McCoy E, McGregor D, Mortelmans K et al (1984) Reproducibility of microbial mutagenicity assays: I. Tests with Salmonella typhimurium and Escherichia coli using a standardized protocol. Environ Mutagen 6(Suppl 2):1–251
Toolaram AP, Kümmerer K, Schneider M (2014) Environmental risk assessment of anti-cancer drugs and their transformation products: a focus on their genotoxicity characterization-state of knowledge and short comings. Mutat Res Rev Mutat Res. https://doi.org/10.1016/j.mrrev.2014.02.001
Quillardet P, Huisman O, D’Ari R, Hofnung M (1982) SOS chromotest, a direct assay of induction of an SOS function in Escherichia coli K-12 to measure genotoxicity. Proc Natl Acad Sci U S A 79:5971–5975
Radman M (1975) SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci 5A:355–367. https://doi.org/10.1007/978-1-4684-2895-7_48
Michel B (2005) After 30 years of study, the bacterial SOS response still surprises us. PLoS Biol 3:e255. https://doi.org/10.1371/journal.pbio.0030255
Matney TS, Nguyen TV, Connor TH, Theiss JC, Dana WJ (1985) Genotoxic classification of anticancer drugs. Teratog Carcinog Mutagen 1(5):319–328. https://doi.org/10.1002/tcm.1770050502
Hannan MA, al-Dakan AA, Hussain SS, Amer MH (1989) Mutagenicity of cisplatin and carboplatin used alone and in combination with four other anticancer drugs. Toxicology 55:183–91
Bouayadi K, Villani G, Salles B (1993) Resistance to cisplatin in an E. coli B/r NalR mutant. Mutat Res 1(294):77–87. https://doi.org/10.1016/0921-8777(93)90060-T
Keller KL, Overbeck-Carrick TL, Beck DJ (2001) Survival and induction of SOS in Escherichia coli treated with cisplatin, UV-irradiation, or mitomycin C are dependent on the function of the RecBC and RecFOR pathways of homologous recombination. Mutat Res 5(486):21–29
Szikriszt B, Póti Á, Pipek O, Krzystanek M, Kanu N, Molnár J et al (2016) A comprehensive survey of the mutagenic impact of common cancer cytotoxics. Genome Biol 17. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4862131. https://doi.org/10.1186/s13059-016-0963-7
Parrella A, Lavorgna M, Criscuolo E, Russo C, Isidori M (2015) Eco-genotoxicity of six anticancer drugs using comet assay in daphnids. J Hazard Mater 9(286):573–580. https://doi.org/10.1016/j.jhazmat.2015.01.012
Papanicolas LE, Gordon DL, Wesselingh SL, Rogers GB (2018) Not just antibiotics: is cancer chemotherapy driving antimicrobial resistance? Trends Microbiol 26:393–400. https://doi.org/10.1016/j.tim.2017.10.009
Ames BN, Gurney EG, Miller JA, Bartsch H (1972) Carcinogens as frameshift mutagens: metabolites and derivatives of 2-acetylaminofluorene and other aromatic amine carcinogens. Proc Natl Acad Sci U S A 69:3128–3132
Cohen SS, I J (1976) Synthesis and the lethality of bleomycin in bacteria. Cancer Res. 1(36):2768–74
Dortet L, Nordmann P, Poirel L (2012) Association of the emerging carbapenemase NDM-1 with a bleomycin resistance protein in Enterobacteriaceae and Acinetobacter baumannii. Antimicrob Agents Chemother 56:1693–1697. https://doi.org/10.1128/AAC.05583-11
Gieringer JH, Wenz AF, Just HM, Daschner FD (1986) Effect of 5-fluorouracil, mitoxantrone, methotrexate, and vincristine on the antibacterial activity of ceftriaxone, ceftazidime, cefotiam, piperacillin, and netilmicin. Chemotherapy 32:418–424
López-Jácome E, Franco-Cendejas R, Quezada H, Morales-Espinosa R, I I, González-Pedrajo B et al (2019) The race between drug introduction and appearance of microbial resistance. Current balance and alternative approaches. Curr Opin Pharmacol 48:48–56. https://doi.org/10.1016/j.coph.2019.04.016
Soo VWC, Kwan BW, Quezada H, Castillo-Juárez I, Pérez-Eretza B, García-Contreras SJ et al (2017) Repurposing of anticancer drugs for the treatment of bacterial infections. Curr Top Med Chem 17:1157–1176. https://doi.org/10.2174/1568026616666160930131737
Stringer AM, Gibson RJ, Bowen JM, Keefe DMK (2009) Chemotherapy-induced modifications to gastrointestinal microflora: evidence and implications of change. [cited 2020 9]. https://www.ingentaconnect.com/content/ben/cdm/2009/00000010/00000001/art00009. https://doi.org/10.2174/138920009787048419
Pačes V, Doskočil J, Šorm F (1968) Incorporation of 5-azacytidine into nucleic acids of Escherichia coli. Biochim Biophys Acta BBA - Nucleic Acids Protein Synth. 161:352–60. https://doi.org/10.1016/0005-2787(68)90113-5
Friedman S, Cheong LC (1984) Effect of 5-azacytidine on deoxyribonucleic acid methylation in Escherichia coli K12. Biochem Pharmacol 15(33):2675–2679. https://doi.org/10.1016/0006-2952(84)90644-0
Peiris V, Oppenheim BA (1993) Antimicrobial activity of cytotoxic drugs may influence isolation of bacteria and fungi from blood cultures. J Clin Pathol 46:1124–1125
Bjedov I, Tenaillon O, Gérard B, Souza V, Denamur E, Radman M et al (2003) Stress-induced mutagenesis in bacteria. Science 30(300):1404–1409. https://doi.org/10.1126/science.1082240
Beaber JW, Hochhut B, Waldor MK (2004) SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature 1(427):72–74. https://doi.org/10.1038/nature02241
Moody MR, Morris MJ, Young VM, Moyé LA, Schimpff SC, Wiernik PH (1978) Effect of two cancer chemotherapeutic agents on the antibacterial activity of three antimicrobial agents. Antimicrob Agents Chemother 14:737–742
Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE et al (2018) Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 29(555):623–628. https://doi.org/10.1038/nature25979
Dortet L, Girlich D, Virlouvet A-L, Poirel L, Nordmann P, Iorga BI et al (2017) Characterization of BRPMBL, the bleomycin resistance protein associated with the carbapenemase NDM. Antimicrob Agents Chemother 61:e02413-e2416. https://doi.org/10.1128/AAC.02413-16
Zwielehner J, Lassl C, Hippe B, Pointner A, Switzeny OJ, Remely M et al (2011) Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS ONE 6:e28654. https://doi.org/10.1371/journal.pone.0028654
Pietri SD, Ingham AC, Frandsen TL, Rathe M, Krych L, Castro-Mejía JL et al (2020) Gastrointestinal toxicity during induction treatment for childhood acute lymphoblastic leukemia: the impact of the gut microbiota. Int J Cancer 147:1953–1962. https://doi.org/10.1002/ijc.32942
Xu X, Zhang X (2015) Effects of cyclophosphamide on immune system and gut microbiota in mice. Microbiol Res 171:97–106. https://doi.org/10.1016/j.micres.2014.11.002
Rashid MU, Rosenborg S, Panagiotidis G, Löfdal KS, Weintraub A, Nord CE (2015) Ecological effect of ceftazidime/avibactam on the normal human intestinal microbiota. Int J Antimicrob Agents 46:60–65. https://doi.org/10.1016/j.ijantimicag.2015.02.027
Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C et al (2015) Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 27(350):1079–1084. https://doi.org/10.1126/science.aad1329
Daillère R, Vétizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V et al (2016) Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 18(45):931–943. https://doi.org/10.1016/j.immuni.2016.09.009
van Vliet MJ, Harmsen HJM, de Bont ESJM, Tissing WJE (2010) The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog 27(6):e1000879. https://doi.org/10.1371/journal.ppat.1000879
Sonis ST (2004) The pathobiology of mucositis. Nat Rev Cancer 4:277–284. https://doi.org/10.1038/nrc1318
Yi M, Jiao D, Qin S, Chu Q, Li A, Wu K (2019) Manipulating gut microbiota composition to enhance the therapeutic effect of cancer immunotherapy. Integr Cancer Ther 1(18):1534735419876351. https://doi.org/10.1177/1534735419876351
Leite JB, Vilela EG, da Gama Torres HO, de Lourdes de Abreu Ferrari M, da Cunha AS (2014) Intestinal permeability in leukemic patients prior to chemotherapy. Rev Bras Hematol E Hemoter 36:409–13. https://doi.org/10.1016/j.bjhh.2014.07.007
De Pietri S, Frandsen TL, Christensen M, Grell K, Rathe M, Müller K (2021) Citrulline as a biomarker of bacteraemia during induction treatment for childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer 68:e28793. https://doi.org/10.1002/pbc.28793
Bow EJ, Meddings JB (2006) Intestinal mucosal dysfunction and infection during remission-induction therapy for acute myeloid leukaemia. Leukemia 20:2087–2092. https://doi.org/10.1038/sj.leu.2404440
Blijlevens NMA, van Land B, Donnelly JP, M’Rabet L, de Pauw BE (2004) Measuring mucosal damage induced by cytotoxic therapy. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 12:227–33. https://doi.org/10.1007/s00520-003-0572-3
Sundström GM, Wahlin A, Nordin-Andersson I, Suhr OB (1998) Intestinal permeability in patients with acute myeloid leukemia. Eur J Haematol 61:250–254
van Vliet MJ, Tissing WJE, Rings EHHM, Koetse HA, Stellaard F, Kamps WA et al (2009) Citrulline as a marker for chemotherapy induced mucosal barrier injury in pediatric patients. Pediatr Blood Cancer 15(53):1188–1194. https://doi.org/10.1002/pbc.22210
Krndija D, Marjou FE, Guirao B, Richon S, Leroy O, Bellaiche Y et al (2019) Active cell migration is critical for steady-state epithelial turnover in the gut. Science 16(365):705–710. https://doi.org/10.1126/science.aau3429
Roy S, Trinchieri G (2017) Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer 17:271–285. https://doi.org/10.1038/nrc.2017.13
Panebianco C, Andriulli A, Pazienza V (2018) Pharmacomicrobiomics: exploiting the drug-microbiota interactions in anticancer therapies. Microbiome [cited 2020 14];6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5964925. https://doi.org/10.1186/s40168-018-0483-7
Ghanem S, Kim CJ, Dutta D, Salifu M, Lim SH. Antimicrobial therapy during cancer treatment: beyond antibacterial effects. J Intern Med [cited 2021 29];n/a. https://onlinelibrary.wiley.com/doi/abs/10.1111/joim.13238. https://doi.org/10.1111/joim.13238
Tlaskalová-Hogenová H, Štěpánková R, Kozáková H, Hudcovic T, Vannucci L, Tučková L et al (2011) The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol 8:110. https://doi.org/10.1038/cmi.2010.67
Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Microbial ecology: human gut microbes associated with obesity. Nature 21(444):1022–1023. https://doi.org/10.1038/4441022a
Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J et al (2009) The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol 9(9):123. https://doi.org/10.1186/1471-2180-9-123
Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci 2(102):11070–11075. https://doi.org/10.1073/pnas.0504978102
Ling Z, Liu X, Jia X, Cheng Y, Luo Y, Yuan L et al (2014) Impacts of infection with different toxigenic Clostridium difficile strains on faecal microbiota in children. Sci Rep 15(4):7485. https://doi.org/10.1038/srep07485
Peterson DA, Frank DN, Pace NR, Gordon JI (2008) Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe 12(3):417–427. https://doi.org/10.1016/j.chom.2008.05.001
Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S et al (2014) Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther 40:409–21. https://doi.org/10.1111/apt.12878
Galloway-Peña JR, Smith DP, Sahasrabhojane P, Ajami NJ, Wadsworth WD, Daver NG et al (2016) The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer 15(122):2186–2196. https://doi.org/10.1002/cncr.30039
Viaud S, Daillère R, Yamazaki T, Lepage P, Boneca I, Goldszmid R et al (2014) Why should we need the gut microbiota to respond to cancer therapies? Oncoimmunology 1(3):e27574. https://doi.org/10.4161/onci.27574
Couzin-Frankel J (2013) Breakthrough of the year 2013. Cancer Immunother Sci 20(342):1432–1433. https://doi.org/10.1126/science.342.6165.1432
Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV et al (2018) Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 5(359):97–103. https://doi.org/10.1126/science.aan4236
Lehouritis P, Cummins J, Stanton M, Murphy CT, McCarthy FO, Reid G et al (2015) Local bacteria affect the efficacy of chemotherapeutic drugs. Sci Rep 29(5):14554. https://doi.org/10.1038/srep14554
Vande Voorde J, Sabuncuoğlu S, Noppen S, Hofer A, Ranjbarian F, Fieuws S et al (2014) Nucleoside-catabolizing enzymes in mycoplasma-infected tumor cell cultures compromise the cytostatic activity of the anticancer drug gemcitabine. J Biol Chem 9(289):13054–13065. https://doi.org/10.1074/jbc.M114.558924
Gao Z, Guo B, Gao R, Zhu Q, Qin H (2015) Microbiota disbiosis is associated with colorectal cancer. Front Microbiol [cited 2020 2];6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4313696. https://doi.org/10.3389/fmicb.2015.00020
Galloway-Peña JR, Jenq RR, Shelburne SA (2017) Can consideration of the microbiome improve antimicrobial utilization and treatment outcomes in the oncology patient? Clin Cancer Res Off J Am Assoc Cancer Res. 23:3263–8. https://doi.org/10.1158/1078-0432.CCR-16-3173
Doré J, Multon M-C, Béhier J-M (2017) Participants of Giens XXXII, Round Table No. 2. The human gut microbiome as source of innovation for health: which physiological and therapeutic outcomes could we expect? Therapie 72:21–38. https://doi.org/10.1016/j.therap.2016.12.007
Mirnezami R, Nicholson J, Darzi A (2012) Preparing for precision medicine. N Engl J Med 9(366):489–491. https://doi.org/10.1056/NEJMp1114866
Morkūnas E, Skiecevičienė J, Kupčinskas J (2020) The impact of modulating the gastrointestinal microbiota in cancer patients. Best Pract Res Clin Gastroenterol 1(48–49):101700. https://doi.org/10.1016/j.bpg.2020.101700
Wallace BD, Wang H, Lane KT, Scott JE, Orans J, Koo JS et al (2010) Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 5(330):831–835. https://doi.org/10.1126/science.1191175
de Gunzburg J, Ghozlane A, Ducher A, Le Chatelier E, Duval X, Ruppé E et al (2018) Protection of the human gut microbiome from antibiotics. J Infect Dis 30(217):628–636. https://doi.org/10.1093/infdis/jix604
Baumgartner M, Pfrunder-Cardozo KR, Hall AR. Microbial community composition interacts with local abiotic conditions to drive colonization resistance in human gut microbiome samples. Proc R Soc B Biol Sci 288:20203106. https://doi.org/10.1098/rspb.2020.3106
Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S et al (2018) Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 174:1388-1405.e21. https://doi.org/10.1016/j.cell.2018.08.041
Gerbitz A, Schultz M, Wilke A, Linde H-J, Schölmerich J, Andreesen R et al (2004) Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood 1(103):4365–4367. https://doi.org/10.1182/blood-2003-11-3769
Wada M, Nagata S, Saito M, Shimizu T, Yamashiro Y, Matsuki T et al (2010) Effects of the enteral administration of Bifidobacterium breve on patients undergoing chemotherapy for pediatric malignancies. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 18:751–9. https://doi.org/10.1007/s00520-009-0711-6
Van de Wiele T, Van den Abbeele P, Ossieur W, Possemiers S, Marzorati M (2015 ) The simulator of the human intestinal microbial ecosystem (SHIME®) [Internet]. In: Verhoeckx K, Cotter P, López-Expósito I, Kleiveland C, Lea T, Mackie A, et al., editors. The Impact of Food Bioactives on Health: in vitro and ex vivo models. Cham (CH): Springer; [cited 2018 17]. http://www.ncbi.nlm.nih.gov/books/NBK500150/
Kumar R, Sood U, Gupta V, Singh M, Scaria J, Lal R (2020) Recent advancements in the development of modern probiotics for restoring human gut microbiome dysbiosis. Indian J Microbiol 1(60):12–25. https://doi.org/10.1007/s12088-019-00808-y
Chen D, Wu J, Jin D, Wang B, Cao H (2019) Fecal microbiota transplantation in cancer management: current status and perspectives. Int J Cancer 145:2021–2031. https://doi.org/10.1002/ijc.32003
Routy B, Chatelier EL, Derosa L, Duong CPM, Alou MT, Daillère R et al (2018) Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 5(359):91–97. https://doi.org/10.1126/science.aan3706
Derrien M, Van Baarlen P, Hooiveld G, Norin E, Müller M, de Vos WM (2011) Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader Akkermansia muciniphila. Front Microbiol 2:166. https://doi.org/10.3389/fmicb.2011.00166
Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R et al (2017) European consensus conference on faecal microbiota transplantation in clinical practice. Gut 1(66):569–580. https://doi.org/10.1136/gutjnl-2016-313017
Zhang F, Luo W, Shi Y, Fan Z, Ji G (2012) Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol 107:1755; author reply p.1755–1756. https://doi.org/10.1038/ajg.2012.251
Khoruts A, Sadowsky MJ (2016) Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol 13:508–516. https://doi.org/10.1038/nrgastro.2016.98
Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L et al (2021) Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 5(371):602–609. https://doi.org/10.1126/science.abb5920
Olesen SW, Leier MM, Alm EJ, Kahn SA (2018) Searching for superstool: maximizing the therapeutic potential of FMT. Nat Rev Gastroenterol Hepatol 15:387–388. https://doi.org/10.1038/s41575-018-0019-4
Kellermayer R (2019) Fecal microbiota transplantation: great potential with many challenges. Transl Gastroenterol Hepatol [cited 2021 26];4. https://tgh.amegroups.com/article/view/5081. https://doi.org/10.21037/tgh.2019.05.10
Malard F, Vekhoff A, Lapusan S, Isnard F, D’incan-Corda E, Rey J et al (2021) Gut microbiota diversity after autologous fecal microbiota transfer in acute myeloid leukemia patients. Nat Commun 12:3084. https://doi.org/10.1038/s41467-021-23376-6
Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF (2017) The microbiota–gut–brain axis in obesity. Lancet Gastroenterol Hepatol 1(2):747–756. https://doi.org/10.1016/S2468-1253(17)30147-4
Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, Kassam Z et al (2019) International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 1(68):2111–2121. https://doi.org/10.1136/gutjnl-2019-319548
Payne AN, Zihler A, Chassard C, Lacroix C (2012) Advances and perspectives in in vitro human gut fermentation modeling. Trends Biotechnol 1(30):17–25. https://doi.org/10.1016/j.tibtech.2011.06.011
Williams Cf, Walton Ge, Jiang L, Plummer S, Garaiova I, Gibson Gr (2015) Comparative analysis of intestinal tract models. Annu Rev Food Sci Technol 6:329–50. https://doi.org/10.1146/annurev-food-022814-015429
Auchtung JM, Robinson CD, Britton RA (2015) Cultivation of stable, reproducible microbial communities from different fecal donors using minibioreactor arrays (MBRAs). Microbiome 30(3):42. https://doi.org/10.1186/s40168-015-0106-5
Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE et al (2015) Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519:92–96. https://doi.org/10.1038/nature14232
Le Bastard Q, Ward T, Sidiropoulos D, Hillmann BM, Chun CL, Sadowsky MJ et al (2018) Fecal microbiota transplantation reverses antibiotic and chemotherapy-induced gut dysbiosis in mice. Sci Rep 18(8):6219. https://doi.org/10.1038/s41598-018-24342-x
Taur Y, Jenq RR, Perales M-A, Littmann ER, Morjaria S, Ling L et al (2014) The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 14(124):1174–1182. https://doi.org/10.1182/blood-2014-02-554725
Taur Y, Coyte K, Schluter J, Robilotti E, Figueroa C, Gjonbalaj M et al (2018) Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci Transl Med 26:10. https://doi.org/10.1126/scitranslmed.aap9489
Acknowledgements
We thank Imane El Meouche for her comments on our manuscript and Anna Hobson for the English editing.
Funding
Claire Amaris Hobson is currently supported by funds from the ARC Foundation (grant no. DOC20190509066) for a PhD. The Q.E.M Team currently works with FRM fundings, EQU201903007848.
Author information
Authors and Affiliations
Contributions
CAHobson, writing and literature research; SBonacorsi, writing; ABaruchel, writing; OTenaillon, writing; ABirgy, writing and literature research.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hobson, C.A., Bonacorsi, S., Baruchel, A. et al. The interplay between anticancer challenges and the microbial communities from the gut. Eur J Clin Microbiol Infect Dis 41, 691–711 (2022). https://doi.org/10.1007/s10096-022-04435-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-022-04435-2