Abstract
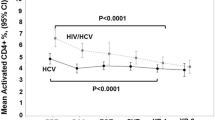
To analyze the modifications of CD4 T cell, CD4/CD8 ratio, and serum levels of soluble CD14 (sCD14) in HIV/HCV-coinfected patients after treatment with direct anti-HCV antiviral agents. Consecutive cases of HIV/HCV-coinfected patients, attended at the University Hospital, who achieved sustained virological responses with interferon-free hepatitis C antiviral drugs, were analyzed. Thirty-five percent of patients (n = 39) had been diagnosed with liver cirrhosis. The evaluation criteria were changes in CD4 T-cell counts and percentages and inflammation (measured by serum sCD14 levels) or immune activation indexes (determined by CD4/CD8 ratio) from beginning anti-HCV therapy to 12 months later. One hundred twelve patients were included (87% male; median age, 54 years; median time from the infection diagnosis, 22 years; previous drug users, 87%). Significant increases in CD4 T cell count and percentage were detected only in individuals without liver cirrhosis. No significant differences in CD4/CD8 ratios or sCD14 levels were observed in patients with or without cirrhosis. The proportion of patients with less than 500 CD4 T cell/mm3 before therapy who achieved more than 500 CD4 T cell/mm3 after it increased only in the group without liver cirrhosis. The finding that CD4 T cell count and percentage were improved only in patients without liver cirrhosis supports the idea that treatment against HCV in HIV/HCV-coinfected patients is needed in the early phases of liver disease.

Similar content being viewed by others
Availability of materials and data
All data generated or analyzed during this study are included in this published article.
References
Berenguer J, Rodríguez E, Miralles P, Von Wichmann MA, López-Aldeguer J, Mallolas J et al (2012) GESIDA HIV/HCV Cohort Study Group. Sustained virological response to interferon plus ribavirin reduces non-liver-related mortality in patients coinfected with HIV and hepatitis C virus. Clin Infect Dis 55:728–736
Mira JA, Rivero-Juárez A, López-Cortés LF, Girón-González JA, Téllez F, de los Santos-Gil I et al (2013) Benefits from sustained virologic response to pegylated interferon plus ribavirin in HIV/hepatitis C virus-coinfected patients with compensated cirrhosis. Clin Infect Dis 56:1646–1653
Berenguer J, Rodríguez-Castellano E, Carrero A, Von Wichmann MA, Montero M, Galindo MJ et al (2017) GESIDA HIV/HCV Cohort Study Group. Eradication of hepatitis C virus and non-liver-related non-acquired immune deficiency syndrome-related events in human immunodeficiency virus/hepatitis C virus coinfection. Hepatology 66:344–356
Kostadinova L, Shive CL, Judge C, Zebrowski E, Compan A, Rife K et al (2016) During hepatitis C virus (HCV) infection and HCV-HIV coinfection, an elevated plasma level of autotaxin is associated with lysophosphatidic acid and markers of immune activation that normalize during interferon-free HCV therapy. J Infect Dis 214:1438–1448
Bas S, Gauthier BR, Spenato U, Stingelin S, Gabay C (2004) CD14 is an acute phase protein. J Immunol 172:4470–4479
Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE et al (2011) Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 203:780–790
Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N et al (2014) Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 210:1228–1238
Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL et al (2014) HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8 T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 10:e1004078
Serrano-Villar S, Perez-Elias MJ, Dronda F, Casado JL, Moreno A, Royuela A et al (2014) Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One 9:e85798
Serrano-Villar S, Gutiérrez C, Vallejo A, Hernández-Novoa B, Díaz L, Abad Fernández M et al (2013) The CD4/CD8 ratio in HIV-infected subjects is independently associated with T-cell activation despite long-term viral suppression. J Infect 66:57–66
Mussini C, Lorenzini P, Cozzi-Lepri A, Lapadula G, Marchetti G, Nicastri E et al (2015) CD4/CD8 ratio normalization and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV 2:e98–e106
Zaegel-Faucher O, Bregigeon S, Cano CE, Obry-Roguet V, Nicolino-Brunet C, Tamalet C et al (2015) Impact of hepatitis C virus coinfection on T-cell dynamics in long-term HIV-suppressors under combined antiretroviral therapy. AIDS 29:1505–1510
Kuniholm MH, OʼBrien TR, Prokunina-Olsson L, Augenbraun M, Plankey M, Karim R et al (2016) Association of hepatitis C virus infection with CD4/CD8 ratio in HIV-positive women. J Acquir Immune Defic Syndr 72:162–170
Potter M, Odueyungbo A, Yang H, Saeed S, Klein MB, Canadian Co-infection Cohort Study Investigators (2010) Impact of hepatitis C viral replication on CD4 T-lymphocyte progression in HIV-HCV coinfection before and after antiretroviral therapy. AIDS 24:1857–1865
Tsiara CG, Nikolopoulos GK, Dimou NL, Bagos PG, Saroglou G, Velonakis E, Hatzakis A (2013) Effect of hepatitis C virus on immunological and virological responses in HIV-infected patients initiating highly active antiretroviral therapy: a meta-analysis. J Viral Hepat 20:715–724
López-Cortés LF, Trujillo-Rodríguez M, Báez-Palomo A, Benmarzouk-Hidalgo OJ, Dominguez-Molina B, Milanés-Guisado Y et al (2018) Eradication of hepatitis C virus (HCV) reduces immune activation, microbial translocation, and the HIV DNA level in HIV/HCV-coinfected patients. J Infect Dis 218:624–632
Márquez M, Romero-Cores P, Montes-Oca M, Martín-Aspas A, Soto-Cárdenas MJ, Guerrero F et al (2015) Immune activation response in chronic HIV-infected patients: influence of hepatitis C virus coinfection. PLoS One 10:e0119568
Centers for Disease Control and Prevention (CDC) (2014) Revised surveillance case definition for HIV infection--United States, 2014. MMWR Recomm Rep 63(RR-03):1–10
Vergara S, Macías J, Rivero A, Gutiérrez-Valencia A, González-Serrano M, Merino D et al (2007) Grupo para el Estudio de las Hepatitis Viricas de la SAEI. The use of transient elastometry for assessing liver fibrosis in patients with HIV and hepatitis C virus coinfection. Clin Infect Dis 45:969–974
Pugh R, Murray-lyon I, Dawson J (1973) Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60:646–649
Mocroft A, Phillips AN, Gatell J, Ledergerber B, Fisher M, Clumeck N et al (2007) Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet 370:407–413
Miller MF, Haley C, Koziel MJ, Rowley CF (2005) Impact of hepatitis C virus on immune restoration in HIV-infected patients who start highly active antiretroviral therapy: a meta-analysis. Clin Infect Dis 41:713–720
Hughes MD, Stein DS, Gundacker HM, Valentine FT, Phair JP, Volberding PA (1994) Within-subject variation in CD4 lymphocyte count in asymptomatic human immunodeficiency virus infection: implications for patient monitoring. J Infect Dis 169:28–36
Hulgan T, Raffanti S, Kheshti A, Blackwell RB, Rebeiro PF, Barkanic G et al (2005) CD4 lymphocyte percentage predicts disease progression in HIV-infected patients initiating highly active antiretroviral therapy with CD4 lymphocyte counts >350 lymphocytes/mm3. J Infect Dis 192:950–957
Moore DM, Hogg RS, Yip B, Craib K, Wood E, Montaner JS (2006) CD4 percentage is an independent predictor of survival in patients starting antiretroviral therapy with absolute CD4 cell counts between 200 and 350 cells/microL. HIV Med 7:383–388
Hull MW, Rollet K, Odueyungbo A, Saeed S, Potter M, Cox J et al (2012) Factors associated with discordance between absolute CD4 cell count and CD4 cell percentage in patients coinfected with HIV and hepatitis C virus. Clin Infect Dis 54:1798–1805
McGovern BH, Golan Y, Lopez M, Pratt D, Lawton A, Moore G et al (2007) The impact of cirrhosis on CD4 T cell counts in HIV-seronegative patients. Clin Infect Dis 44:431–437
Marchetti G, Cozzi-Lepri A, Merlini E, Bellistrì GM, Castagna A, Galli M et al (2011) Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4 cell count. AIDS 25:1385–1394
Mendez-Lagares G, Romero-Sanchez MC, Ruiz-Mateos E, Genebat M, Ferrando-Martínez S, Muñoz-Fernández MÁ et al (2013) Long-term suppressive combined antiretroviral treatment does not normalize the serum level of soluble CD14. J Infect Dis 207:1221–1225
Márquez M, Fernández Gutiérrez del Álamo C, Girón-González JA (2016) Gut epithelial barrier dysfunction in human immunodeficiency virus-hepatitis C virus coinfected patients: influence on innate and acquired immunity. World J Gastroenterol 22:1433–1448
Lens S, Alvarado-Tapias E, Mariño Z, Londoño MC, LLop E, Martinez J et al (2017) Effects of all-oral anti-viral therapy on HVPG and systemic hemodynamics in patients with hepatitis C virus-associated cirrhosis. Gastroenterology 153:1273–1283
Margolick JB, Muñoz A, Donnenberg AD, Park LP, Galai N, Giorgi JV et al (1995) Failure of T-cell homeostasis preceding AIDS in HIV-1 infection. The Multicenter AIDS Cohort Study. Nat Med 1:674–680
Brites-Alves C, Netto EM, Brites C (2015) Coinfection by hepatitis C is strongly associated with abnormal CD4/CD8 ratio in HIV patients under stable ART in Salvador, Brazil. J Immunol Res 2015:174215
Saracino A, Bruno G, Scudeller L, Ladisa N, de Gennaro N, Allegrini M et al (2016) CD4 and CD4/CD8 ratio progression in HIV-HCV infected patients after achievement of SVR. J Clin Virol 81:94–99
Acknowledgements
We acknowledge to Adrianne E. Jones for technical assistance with the English language.
Funding
This work was supported by a grant of the Secretaría General de Investigación, Desarrollo e Innovación en Salud, Junta de Andalucía, Iniciativa Territorial Integrada 2014-2020 para la provincia de Cádiz (No PI-0076-2017), Spain. Co-financed by FEDER (Fondo Europeo de Desarrollo Regional).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: José-Antonio Girón-Ortega; Mercedes Márquez-Coello; Sara Cuesta Sancho; José-Antonio Girón-González
Acquisition of data: Mercedes Márquez-Coello; Daniel Guitérrez Saborido, Ana Arizcorreta, Sara Cuesta Sancho; José-Antonio Girón-González
Analysis and interpretation of data: José-Antonio Girón-Ortega; Mercedes Márquez-Coello; Sara Cuesta Sancho; José-Antonio Girón-González
Article drafting: José-Antonio Girón-Ortega; Mercedes Márquez-Coello; Sara Cuesta Sancho; José-Antonio Girón-González.
All authors contributed to the conception of the study and critical revision of the manuscript and saw and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The ethical research committee of Hospital Puerta del Mar (Cádiz, Spain) approved the project.
Consent to participate and consent to publish
Informed consent was obtained from all patients for being included in the study.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Girón-Ortega, JA., Márquez-Coello, M., Gutiérrez-Saborido, D. et al. Modifications of CD4 T cells, CD4/CD8 ratio and serum levels of soluble CD14 in HIV-HCV-coinfected patients after sustained HCV response induced by direct-acting antiviral agents: influence of liver cirrhosis. Eur J Clin Microbiol Infect Dis 40, 1863–1871 (2021). https://doi.org/10.1007/s10096-021-04237-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-021-04237-y