Abstract
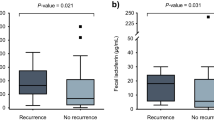
Fecal calprotectin (fCPT) has been used as a surrogate marker for assessment of intestinal inflammation. We explore the utility of fCPT values as a diagnostic aid in cancer patients with suspected Clostridium difficile infection (CDI). A total of 232 stool specimens submitted for GeneXpert C. difficile PCR testing were included in the study. All specimens were tested for fCPT and toxin/GDH antigens. Clinical severity of CDI cases was determined by the IDSA/SHEA criteria. Significant differences of median fCPT values between CDI (n = 117, Median 183.6 μg/g) and non-CDI (n = 115, 145.6 μg/g, p = 0.006) patients were seen. In CDI patents, significantly lower fCPT values were found in patients with mild to moderate (n = 95, 182.1 μg/g) than those with severe and severe to complicated (n = 22, 218.5 μg/g, p = 0.014) scores, and among those that were toxin positive (n = 24, 200.2 μg/g) vs. toxin negative (n = 86, 182.8 μg/g, p = 0.044). Despite this overall trend, wide variations in fCPT values were found in all categories examined. A logistic regression analysis revealed that the fCPT values correlated independently with the severity of clinical manifestations (OR = 2.021, 95%CI = 1.132–3.608); however, it did not correlate with other clinical outcomes. Our study findings show that high fecal calprotectin levels correlate with toxin-positive and clinically severe CDI; however, wide variations in individual measurements preclude establishment of reliable cut-offs for routine diagnostic use in cancer patients.
Similar content being viewed by others
References
Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC (2015) Burden of Clostridium difficile infection in the United States. N Engl J Med 372(9):825–834
Dominguez SR, Dolan SA, West K, Dantes RB, Epson E, Friedman D, Littlehorn CA, Arms LE, Walton K, Servetar E, Frank DN, Kotter CV, Dowell E, Gould CV, Hilden JM, Todd JK (2014) High colonization rate and prolonged shedding of Clostridium difficile in pediatric oncology patients. Clin Infect Dis 59(3):401–403
Furuya-Kanamori L, Marquess J, Yakob L, Riley TV, Paterson DL, Foster NF, Huber CA, Clements AC (2015) Asymptomatic Clostridium difficile colonization: epidemiology and clinical implications. BMC Infect Dis 15:516
Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, Turgeon N, Toye B, Beaudoin A, Frost EH, Gilca R, Brassard P, Dendukuri N, Beliveau C, Oughton M, Brukner I, Dascal A (2011) Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med 365(18):1693–1703
McFarland LV, Ozen M, Dinleyici EC, Goh S (2016) Comparison of pediatric and adult antibiotic-associated diarrhea and Clostridium difficile infections. World J Gastroenterol 22(11):3078–3104
Bagdasarian N, Rao K, Malani PN (2015) Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA 313(4):398–408
Sammons JS, Toltzis P (2015) Pitfalls in diagnosis of pediatric Clostridium difficile infection. Infect Dis Clin N Am 29(3):465–476
Planche TD, Davies KA, Coen PG, Finney JM, Monahan IM, Morris KA, O’Connor L, Oakley SJ, Pope CF, Wren MW, Shetty NP, Crook DW, Wilcox MH (2013) Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C difficile infection. Lancet Infect Dis 13(11):936–945
Polage CR, Gyorke CE, Kennedy MA, Leslie JL, Chin DL, Wang S, Nguyen HH, Huang B, Tang YW, Lee LW, Kim K, Taylor S, Romano PS, Panacek EA, Goodell PB, Solnick JV, Cohen SH (2015) Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 175(11):1792–1801
Peng Z, Ling L, Stratton CW, Li C, Polage CR, Wu B, Tang YW (2018) Advances in the diagnosis and treatment of Clostridium difficile infections. Emerg Microb Infect 7(1):15
Otto CC, Shuptar SL, Milord P, Essick CJ, Nevrekar R, Granovsky SL, Seo SK, Babady NE, Martin SC, Tang YW, Pessin MS (2015) Reducing unnecessary and duplicate ordering for ovum and parasite examinations and Clostridium difficile PCR in immunocompromised patients by using an alert at the time of request in the order management system. J Clin Microbiol 53(8):2745–2748
Kamboj M, Babady NE, Marsh JW, Schlackman JL, Son C, Sun J, Eagan J, Tang YW, Sepkowitz K (2014) Estimating risk of C. difficile transmission from PCR positive but cytotoxin negative cases. PLoS One 9(2):e88262
Lundberg JO, Hellstrom PM, Fagerhol MK, Weitzberg E, Roseth AG (2005) Technology insight: calprotectin, lactoferrin and nitric oxide as novel markers of inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol 2(2):96–102
Calafat M, Cabre E, Manosa M, Lobaton T, Marin L, Domenech E (2015) High within-day variability of fecal calprotectin levels in patients with active ulcerative colitis: what is the best timing for stool sampling? Inflamm Bowel Dis 21(5):1072–1076
Lasson A, Stotzer PO, Ohman L, Isaksson S, Sapnara M, Strid H (2015) The intra-individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J Crohn’s Colitis 9(1):26–32
Swale A, Miyajima F, Roberts P, Hall A, Little M, Beadsworth MB, Beeching NJ, Kolamunnage-Dona R, Parry CM, Pirmohamed M (2014) Calprotectin and lactoferrin faecal levels in patients with Clostridium difficile infection (CDI): a prospective cohort study. PLoS One 9(8):e106118
Usacheva EA, Jin J-P, Peterson LR (2016) Host response to Clostridium difficile infection: diagnostics and detection. J Glob Antimicrob Resist 7:93–101
Kim J, Kim H, Oh HJ, Kim HS, Hwang YJ, Yong D, Jeong SH, Lee K (2017) Fecal calprotectin level reflects the severity of Clostridium difficile infection. Ann Lab Med 37(1):53–57
Peretz A, Tkhawkho L, Pastukh N, Brodsky D, Halevi CN, Nitzan O (2016) Correlation between fecal calprotectin levels, disease severity and the hypervirulent ribotype 027 strain in patients with Clostridium difficile infection. BMC Infect Dis 16:309
Popiel KY, Gheorghe R, Eastmond J, Miller MA (2015) Usefulness of adjunctive fecal calprotectin and serum procalcitonin in individuals positive for Clostridium difficile toxin gene by PCR assay. J Clin Microbiol 53(11):3667–3669
Rao K, Santhosh K, Mogle JA, Higgins PD, Young VB (2016) Elevated fecal calprotectin associates with adverse outcomes from Clostridium difficile infection in older adults. Infect Dis (Lond) 48(9):663–669
Whitehead SJ, Shipman KE, Cooper M, Ford C, Gama R (2014) Is there any value in measuring faecal calprotectin in Clostridium difficile positive faecal samples? J Med Microbiol 63(Pt 4):590–593
Babady NE, Stiles J, Ruggiero P, Khosa P, Huang D, Shuptar S, Kamboj M, Kiehn TE (2010) Evaluation of the Cepheid Xpert Clostridium difficile Epi assay for diagnosis of Clostridium difficile infection and typing of the NAP1 strain at a cancer hospital. J Clin Microbiol 48(12):4519–4524
Quinn CD, Sefers SE, Babiker W, He Y, Alcabasa R, Stratton CW, Carroll KC, Tang YW (2010) C. Diff Quik Chek complete enzyme immunoassay provides a reliable first-line method for detection of Clostridium difficile in stool specimens. J Clin Microbiol 48(2):603–605
Kronborg O, Ugstad M, Fuglerud P, Johne B, Hardcastle J, Scholefield JH, Vellacott K, Moshakis V, Reynolds JR (2000) Faecal calprotectin levels in a high risk population for colorectal neoplasia. Gut 46(6):795–800
Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH (2010) Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 31(5):431–455
Huang B, Jin D, Zhang J, Sun JY, Wang X, Stiles J, Xu X, Kamboj M, Babady NE, Tang YW (2014) Real-time cellular analysis coupled with a specimen enrichment accurately detects and quantifies Clostridium difficile toxins in stool. J Clin Microbiol 52(4):1105–1111. https://doi.org/10.1128/JCM.02601-02613
Kristensen V, Malmstrom GH, Skar V, Roseth A, Moum B (2016) Clinical importance of faecal calprotectin variability in inflammatory bowel disease: intra-individual variability and standardisation of sampling procedure. Scand J Gastroenterol 51(5):548–555
Rodriguez C, Van Broeck J, Taminiau B, Delmee M, Daube G (2016) Clostridium difficile infection: early history, diagnosis and molecular strain typing methods. Microb Pathog 97:59–78
Sewell B, Rees E, Thomas I, Ch’ng CL, Isaac M, Berry N (2014) Cost and impact on patient length of stay of rapid molecular testing for Clostridium difficile. Infect Dis Ther 3(2):281–293
Dubberke ER (2010) Prevention of healthcare-associated Clostridium difficile infection: what works? Infect Control Hosp Epidemiol 31(Suppl 1):S38–S41
Dubberke ER, Haslam DB, Lanzas C, Bobo LD, Burnham CA, Grohn YT, Tarr PI (2011) The ecology and pathobiology of Clostridium difficile infections: an interdisciplinary challenge. Zoonoses Public Health 58(1):4–20
Dabritz J, Musci J, Foell D (2014) Diagnostic utility of faecal biomarkers in patients with irritable bowel syndrome. World J Gastroenterol 20(2):363–375
Dhaliwal A, Zeino Z, Tomkins C, Cheung M, Nwokolo C, Smith S, Harmston C, Arasaradnam RP (2015) Utility of faecal calprotectin in inflammatory bowel disease (IBD): what cut-offs should we apply? Front Gastroenterol 6(1):14–19
Kawashima K, Ishihara S, Yuki T, Fukuba N, Oshima N, Kazumori H, Sonoyama H, Yamashita N, Tada Y, Kusunoki R, Oka A, Mishima Y, Moriyama I, Kinoshita Y (2016) Fecal calprotectin level correlated with both endoscopic severity and disease extent in ulcerative colitis. BMC Gastroenterol 16:47
Mosli MH, Zou G, Garg SK, Feagan SG, MacDonald JK, Chande N, Sandborn WJ, Feagan BG (2015) C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 110(6):802–819 quiz 820
Darkoh C, Turnwald BP, Koo HL, Garey KW, Jiang ZD, Aitken SL, DuPont HL (2014) Colonic immunopathogenesis of Clostridium difficile infections. Clin Vaccine Immunol 21(4):509–517
Acknowledgments
We thank laboratory staff in the Clinical Microbiology Service at MSKCC for their excellent assistance.
Funding
This study was supported in part by the NIH/NCI Cancer Center Support Grant P30 (CA008748).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
Waive approved.
Conflict of interest
The Phical Test was provided free of charge. No other reported conflicts.
Ethical approval
This study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board (WA0477-15).
Rights and permissions
About this article
Cite this article
He, T., Kaplan, S.E., Gomez, L.A. et al. Fecal calprotectin concentrations in cancer patients with Clostridium difficile infection. Eur J Clin Microbiol Infect Dis 37, 2341–2346 (2018). https://doi.org/10.1007/s10096-018-3381-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-3381-9