Abstract
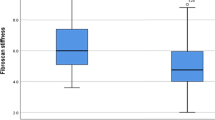
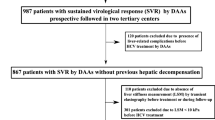
The purpose of this investigation was to evaluate the impact of liver stiffness (LS) on the response to direct-acting antiviral (DAA)-based therapy against hepatitis C virus (HCV) infection in cirrhotic patients. Those patients included in two Spanish prospective cohorts of patients receiving therapy based on at least one DAA, who showed a baseline LS ≥ 12.5 kPa and who had reached the scheduled time point for sustained virological response evaluation 12 weeks after completing therapy (SVR12) were analysed. Pegylated interferon/ribavirin-based therapy plus an HCV NS3/4A protease inhibitor (PR-PI group) was administered to 198 subjects, while 146 received interferon-free regimens (IFN-free group). The numbers of patients with SVR12 according to an LS < 21 kPa versus ≥21 kPa were 59/99 (59.6%) versus 46/99 (46.5%) in the PR-PI group (p = 0.064) and 41/43 (95.3%) versus 90/103 (87.4%) in the IFN-free group (p = 0.232). Corresponding figures for the relapse rates in those who presented end-of-treatment response (ETR) were 3/62 (4.8%) versus 10/56 (17.9%, p = 0.024) and 1/42 (2.4%) versus 8/98 (8.2%, p = 0.278), respectively. In a multivariate analysis adjusted for age, sex and use of interferon, a baseline LS ≥ 21 kPa was identified as an independent predictor of relapse [adjusted odds ratio, AOR (95% confidence interval, CI): 4.228 (1.344–13.306); p = 0.014] in those patients with ETR. LS above 21 kPa is associated with higher rates of relapse to DAA-based therapy in HCV-infected patients with cirrhosis in clinical practice. LS could help us to tailor the duration and composition of DAA-based combinations in cirrhotic subjects, in order to minimise the likelihood of relapse.


Similar content being viewed by others
References
Bruno S, Shiffman ML, Roberts SK et al (2010) Efficacy and safety of peginterferon alfa-2a (40KD) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology 51:388–397
Mira JA, García-Rey S, Rivero A et al (2012) Response to pegylated interferon plus ribavirin among HIV/hepatitis C virus-coinfected patients with compensated liver cirrhosis. Clin Infect Dis 55:1719–1726
Poordad F, McCone J Jr, Bacon BR et al (2011) Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 364:1195–1206
Bacon BR, Gordon SC, Lawitz E et al (2011) Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med; 364:1207–1217
Jacobson IM, McHutchison JG, Dusheiko G (2011) Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 364:2405–2416
Zeuzem S, Andreone P, Pol S et al (2011) Telaprevir for retreatment of HCV infection. N Engl J Med 364:2417–2428
Jacobson IM, Dore GJ, Foster GR et al (2014) Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 384:403–413
Manns M, Marcellin P, Poordad F et al (2014) Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 384:414–426
Zeuzem S, Berg T, Gane E et al (2014) Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a phase IIb trial. Gastroenterology 146:430–441.e6
Forns X, Lawitz E, Zeuzem S et al (2014) Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology 146:1669–1679.e3
Afdhal N, Zeuzem S, Kwo P et al (2014) Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 370:1889–1898
Naggie S, Cooper C, Saag M et al (2015) Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 373:705–713
Wyles DL, Ruane PJ, Sulkowski MS et al (2015) Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 373:714–725
Afdhal N, Reddy KR, Nelson DR et al (2014) Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 370:1483–1493
Nelson DR, Cooper JN, Lalezari JP et al (2015) All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 61:1127–1135
Foster GR, Pianko S, Cooper C et al (2015) Sofosbuvir + peginterferon/ribavirin for 12 weeks vs sofosbuvir + ribavirin for 16 or 24 weeks in genotype 3 HCV infected patients and treatment-experienced cirrhotic patients with genotype 2 HCV: the BOSON study. In: Proceedings of the 50th Annual Meeting of the European Association for the Study of the Liver, Vienna, Austria. 22–26 April 2015. Abstract L05
Documento de consenso del Grupo Español para el Estudio de la Hepatitis (GEHEP) sobre el tratamiento de la hepatitis C. Available online at: http://seimc.org/grupodeestudio.php?Grupo=GEHEP&mn_Grupoid=14&mn_MP=512&mn_MS=513. Accessed 10 October 2016
European Association for the Study of the Liver (EASL). EASL Recommendations on Treatment of Hepatitis C 2015. Available online at: http://www.easl.eu/medias/cpg/HEPC-2015/Full-report.pdf. Accessed 10 October 2016
Sandrin L, Fourquet B, Hasquenoph JM et al (2003) Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 29:1705–1713
Castéra L, Vergniol J, Foucher J et al (2005) Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 128:343–350
Vergara S, Macías J, Rivero A et al (2007) The use of transient elastometry for assessing liver fibrosis in patients with HIV and hepatitis C virus coinfection. Clin Infect Dis 45:969–974
Pineda JA, Recio E, Camacho A et al (2009) Liver stiffness as a predictor of esophageal varices requiring therapy in HIV/hepatitis C virus-coinfected patients with cirrhosis. J Acquir Immune Defic Syndr 51:445–459
Vizzutti F, Arena U, Romanelli RG et al (2007) Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology 45:1290–1297
Mandorfer M, Kozbial K, Schwabl P et al (2016) Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol 65:692–699
Merchante N, Rivero-Juárez A, Téllez F et al (2012) Liver stiffness predicts clinical outcome in human immunodeficiency virus/hepatitis C virus-coinfected patients with compensated liver cirrhosis. Hepatology 56:228–238
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35
Lawitz E, Matusow G, DeJesus E et al (2016) Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: a phase 3 study (OPTIMIST-2). Hepatology 64:360–369
Mauss S, Schewe K, Rockstroh JK et al (2015) Sofosbuvir-based treatments for patients with hepatitis C virus (HCV) mono-infection and human immunodeficiency virus (HIV)–HCV co-infection with genotype 1 and 4 in clinical practice—results from the GErman hepatitis C COhort (GECCO). In: Proceedings of the 66th Annual Meeting of the American Association for the Study of Liver Diseases, San Francisco, CA, USA, 13–17 November 2015. Abstract 1156
Sulkowski M, Hezode C, Gerstoft J et al (2015) Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet 385:1087–1097
Poveda E, Wyles DL, Mena A et al (2014) Update on hepatitis C virus resistance to direct-acting antiviral agents. Antiviral Res 108:181–191
Poordad F, Hezode C, Trinh R et al (2014) ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 370:1973–1982
Reddy KR, Bourlière M, Sulkowski M et al (2015) Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: an integrated safety and efficacy analysis. Hepatology 62:79–86
Ferenci P, Bernstein D, Lalezari J et al (2014) ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med 370:1983–1992
Nelson DR, Cooper JN, Lalezari JP et al (2015) All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 61:1127–1135
Leroy V, Angus P, Bronowicki JP et al (2015) All-oral treatment with daclatasvir (DCV) plus sofosbuvir (SOF) plus ribavirin (RBV) for 12 or 16 weeks in HCV genotype (GT) 3-infected patients with advanced fibrosis or cirrhosis: the ALLY-3+ phase 3 study. In: Proceedings of the 66th Annual Meeting of the American Association for the Study of Liver Diseases, San Francisco, CA, USA, 13–17 November 2015. Abstract LB-3
Reddy KR, Bourlière M, Sulkowski M et al (2015) Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: an integrated safety and efficacy analysis. Hepatology 62:79–86
Bureau C, Metivier S, Peron JM et al (2008) Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther 27:1261–1268
Reiberger T, Rutter K, Ferlitsch A et al (2011) Portal pressure predicts outcome and safety of antiviral therapy in cirrhotic patients with hepatitis C virus infection. Clin Gastroenterol Hepatol 9:602–608.e1
Mandorfer M, Kozbial K, Freissmuth C et al (2015) Interferon-free regimens for chronic hepatitis C overcome the effects of portal hypertension on virological responses. Aliment Pharmacol Ther 42:707–718
Garcia-Tsao G, Friedman S, Iredale J, Pinzani M (2010) Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology 51:1445–1449
Merchante N, Rivero-Juárez A, Téllez F et al (2016) Liver stiffness predicts variceal bleeding in HIV/HCV-coinfected patients with compensated cirrhosis. AIDS (in press)
Charlton M, Everson GT, Flamm SL et al (2015) Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 149:649–659
Poordad F, Schiff ER, Vierling JM et al (2016) Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology 63:1493–1505
Manns M, Samuel D, Gane EJ et al (2016) Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis 16:685–697
Curry MP, O’Leary JG, Bzowej N et al (2015) Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med 373:2618–2628
Acknowledgements
Other members of the GEHEP-SEIMC/HEPAVIR Group are: Helena Albendín, María Remedios Alemán, María del Mar Alonso, Victor Asensi, María José Blanco, Javier Borrallo, Rebeca Cabo, Ángela Camacho, Mario Frías Casas, Ángeles Castro, Antonio Collado, Sandra Cuellar, Francisca Cuenca, Marcial Delgado, Carlos Dueñas, Elisa Fernández, Carlos Galera, María Carmen Gálvez, Dácil García, Paloma Geijo Martínez, Ana Gómez, Juan Luis Gómez, Félix Gutiérrez, José Hernández, Jehovana Hernández, Jara Llenas-García, María Mancebo, José María Martín, Lorena Martínez, Rosa Martínez-Álvarez, Onofre Martínez Madrid, María del Mar Masiá, Nicolás Merchante, Patricia Monje, Marta Montero-Alonso, Rocío Nuñez, Guillermo Ojeda, Sergio Padilla, Catarina Robledano, Ricardo Pelazas, Elisabet Pérez, Inés Pérez-Camacho, Montserrat Pérez-Pérez, Berta Pernas, José Joaquín Portu, Miguel Raffo, Luis M. Real, Gabriel Reina, Sergio Reus, María José Ríos, Antonio Rivero, Joaquín Portilla, Purificación Rubio, Pablo Saíz de la Hoya, Ignacio de los Santos-Gil, Jesús Santos, Miriam Serrano, Marta Suárez-Santamaría, Francisco Téllez, Carla Toyas, Francisco Jesús Vera-Méndez, Antonio Vergara, David Vinuesa García.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
This work has been partially funded by the RD12/0017/0012 project as part of the Plan Nacional R+D+I and cofinanced by ISCIII-Subdirección General de Evaluación, the Fondo Europeo de Desarrollo Regional (FEDER) and the Consejería de Salud of the Junta de Andalucía (grant numbers AC-0095-2013 and PI-0492-2012). K.N. is the recipient of a Miguel Servet research grant from the Instituto de Salud Carlos III (grant number CP13/00187). A.R.-J. is the recipient of a post-doctoral perfection grant from the Consejería de Salud of the Junta de Andalucía (grant number RH-0024/2013). J.M. is the recipient of a grant from the Servicio Andaluz de Salud of the Junta de Andalucía (grant number B-0037). J.A.P. is the recipient of an intensification grant from the Instituto de Salud Carlos III (grant number Programa-I3SNS).
Conflict of interest
K.N. has received lecture fees from Janssen-Cilag, Roche, Bristol-Meyers Squibb and Merck Sharp & Dohme, research support from Janssen-Cilag, Bristol-Meyers Squibb, Merck Sharp & Dohme, Gilead Sciences and Abbott Pharmaceuticals and travel expenses from Janssen-Cilag, ViiV Healthcare, Roche, Bristol-Meyers Squibb and Merck Sharp & Dohme. A.R.-J. has received lecture fees from Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer, ViiV Healthcare and Roche. J.M. has been an investigator in clinical trials supported by Roche, Bristol-Myers Squibb and Abbott Pharmaceuticals and has received lecture fees from Roche, Gilead Sciences, Boehringer Ingelheim and Bristol-Myers Squibb and consulting fees from Boehringer Ingelheim, Bristol-Myers Squibb, Merck Sharp & Dohme and Schering-Plough. R.G. reports having received lecture fees from Roche, Gilead Sciences, Janssen Cilag and Merck Sharp & Dohme. He has received consultancy fees from Janssen Cilag and Abbvie. M.M. reports having received consulting fees from Gilead Sciences and Janssen-Cilag and lecture fees from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck-Sharp & Dome and ViiV Healthcare. D.M. reports having received consultancy fees from Janssen Cilag, Merck Sharp & Dohme, Bristol-Myers Squibb, Gilead Sciences, Abbvie and ViiV Healthcare. J.C. has received consultancy fees from Janssen Cilag, Bristol-Myers Squibb and Gilead Sciences and lecture fees from Janssen Cilag, Bristol-Myers Squibb, Gilead Sciences, ViiV Healthcare and Merck Sharp & Dohme. M.O. has received lecture fees from Abbvie, ViiV Healthcare, Gilead Sciences, Janssen-Cilag, Bristol-Myers Squibb and Merck Sharp & Dohme. J.A.P. reports having received consulting fees from GlaxoSmithKline, Bristol-Myers Squibb, Abbott Pharmaceuticals, Gilead Sciences, Merck Sharp & Dohme, Schering-Plough, Janssen-Cilag and Boehringer Ingelheim. He has received research support from GlaxoSmithKline, Roche, Bristol-Myers Squibb, Schering-Plough, Abbott Pharmaceuticals and Boehringer Ingelheim and has received lecture fees from GlaxoSmithKline, Roche, Abbott Pharmaceuticals, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, Janssen-Cilag, Boehringer Ingelheim and Schering-Plough. All other authors: none to declare.
Ethical approval
The study was designed and performed according to the Helsinki declaration and was approved by the Ethics Committee of the Valme University Hospital (Seville, Spain).
Informed consent
All patients gave their written informed consent before being included in the study.
Rights and permissions
About this article
Cite this article
Neukam, K., Morano-Amado, L.E., Rivero-Juárez, A. et al. Liver stiffness predicts the response to direct-acting antiviral-based therapy against chronic hepatitis C in cirrhotic patients. Eur J Clin Microbiol Infect Dis 36, 853–861 (2017). https://doi.org/10.1007/s10096-016-2871-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2871-x