Abstract
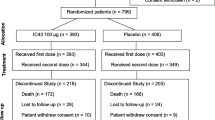
The fully human anti-lipopolysaccharide (LPS) immunoglobulin M (IgM) monoclonal antibody panobacumab was developed as an adjunctive immunotherapy for the treatment of O11 serotype Pseudomonas aeruginosa infections. We evaluated the potential clinical efficacy of panobacumab in the treatment of nosocomial pneumonia. We performed a post-hoc analysis of a multicenter phase IIa trial (NCT00851435) designed to prospectively evaluate the safety and pharmacokinetics of panobacumab. Patients treated with panobacumab (n = 17), including 13 patients receiving the full treatment (three doses of 1.2 mg/kg), were compared to 14 patients who did not receive the antibody. Overall, the 17 patients receiving panobacumab were more ill. They were an average of 72 years old [interquartile range (IQR): 64–79] versus an average of 50 years old (IQR: 30–73) (p = 0.024) and had Acute Physiology and Chronic Health Evaluation II (APACHE II) scores of 17 (IQR: 16–22) versus 15 (IQR: 10–19) (p = 0.043). Adjunctive immunotherapy resulted in an improved clinical outcome in the group receiving the full three-course panobacumab treatment, with a resolution rate of 85 % (11/13) versus 64 % (9/14) (p = 0.048). The Kaplan–Meier survival curve showed a statistically significantly shorter time to clinical resolution in this group of patients (8.0 [IQR: 7.0–11.5] versus 18.5 [IQR: 8–30] days in those who did not receive the antibody; p = 0.004). Panobacumab adjunctive immunotherapy may improve clinical outcome in a shorter time if patients receive the full treatment (three doses). These preliminary results suggest that passive immunotherapy targeting LPS may be a complementary strategy for the treatment of nosocomial O11 P. aeruginosa pneumonia.


Similar content being viewed by others
References
Eggimann P, Pittet D (2001) Infection control in the ICU. Chest 120(6):2059–2093
Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K; EPIC II Group of Investigators (2009) International study of the prevalence and outcomes of infection in intensive care units. JAMA 302(21):2323–2329
Tabah A, Koulenti D, Laupland K, Misset B, Valles J, Bruzzi de Carvalho F, Paiva JA, Cakar N, Ma X, Eggimann P, Antonelli M, Bonten MJ, Csomos A, Krueger WA, Mikstacki A, Lipman J, Depuydt P, Vesin A, Garrouste-Orgeas M, Zahar JR, Blot S, Carlet J, Brun-Buisson C, Martin C, Rello J, Dimopoulos G, Timsit JF (2012) Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med 38(12):1930–1945
Harris AD, Johnson JK, Thom KA, Morgan DJ, McGregor JC, Ajao AO, Moore AC, Comer AC, Furuno JP (2011) Risk factors for development of intestinal colonization with imipenem-resistant Pseudomonas aeruginosa in the intensive care unit setting. Infect Control Hosp Epidemiol 32(7):719–722
Ong DS, Jongerden IP, Buiting AG, Leverstein-van Hall MA, Speelberg B, Kesecioglu J, Bonten MJ (2011) Antibiotic exposure and resistance development in Pseudomonas aeruginosa and Enterobacter species in intensive care units. Crit Care Med 39(11):2458–2463
Kollef MH, Silver P, Murphy DM, Trovillion E (1995) The effect of late-onset ventilator-associated pneumonia in determining patient mortality. Chest 108(6):1655–1662
Trouillet JL, Vuagnat A, Combes A, Kassis N, Chastre J, Gibert C (2002) Pseudomonas aeruginosa ventilator-associated pneumonia: comparison of episodes due to piperacillin-resistant versus piperacillin-susceptible organisms. Clin Infect Dis 34(8):1047–1054
Parker CM, Kutsogiannis J, Muscedere J, Cook D, Dodek P, Day AG, Heyland DK; Canadian Critical Care Trials Group (2008) Ventilator-associated pneumonia caused by multidrug-resistant organisms or Pseudomonas aeruginosa: prevalence, incidence, risk factors, and outcomes. J Crit Care 23(1):18–26
Peña C, Gómez-Zorrilla S, Oriol I, Tubau F, Dominguez MA, Pujol M, Ariza J (2013) Impact of multidrug resistance on Pseudomonas aeruginosa ventilator-associated pneumonia outcome: predictors of early and crude mortality. Eur J Clin Microbiol Infect Dis 32(3):413–420
Boucher HW, Talbot GH, Benjamin DK Jr, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D; Infectious Diseases Society of America (2013) 10 × ’20 Progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 56(12):1685–1694
Chastre J, Luyt CE (2010) Other therapeutic modalities and practices: implications for clinical trials of hospital-acquired or ventilator-associated pneumonia. Clin Infect Dis 51(Suppl 1):S54–S58
François B, Luyt CE, Dugard A, Wolff M, Diehl JL, Jaber S, Forel JM, Garot D, Kipnis E, Mebazaa A, Misset B, Andremont A, Ploy MC, Jacobs A, Yarranton G, Pearce T, Fagon JY, Chastre J (2012) Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized, double-blind, placebo-controlled trial. Crit Care Med 40(8):2320–2326
Hsu JL, Safdar N (2011) Polyclonal immunoglobulins and hyperimmune globulins in prevention and management of infectious diseases. Infect Dis Clin North Am 25(4):773–788
[No authors listed] (1992) Prophylactic intravenous administration of standard immune globulin as compared with core-lipopolysaccharide immune globulin in patients at high risk of postsurgical infection. The Intravenous Immunoglobulin Collaborative Study Group. N Engl J Med 327(4):234–240
Laupland KB, Kirkpatrick AW, Delaney A (2007) Polyclonal intravenous immunoglobulin for the treatment of severe sepsis and septic shock in critically ill adults: a systematic review and meta-analysis. Crit Care Med 35(12):2686–2692
Abraham E (1998) Cytokine modifiers: pipe dream or reality? Chest 113(3 Suppl):224S–227S
Ter Meulen J (2011) Monoclonal antibodies in infectious diseases: clinical pipeline in 2011. Infect Dis Clin North Am 25(4):789–802
Rotschild M, Elias N, Berkowitz D, Pollak S, Shinawi M, Beck R, Bentur L (2005) Autoantibodies against bactericidal/permeability-increasing protein (BPI-ANCA) in cystic fibrosis patients treated with azithromycin. Clin Exp Med 5(2):80–85
Sawada S, Kawamura T, Masuho Y (1987) Immunoprotective human monoclonal antibodies against five major serotypes of Pseudomonas aeruginosa. J Gen Microbiol 133(12):3581–3590
Lang AB, Schaad UB, Rüdeberg A, Wedgwood J, Que JU, Fürer E, Cryz SJ Jr (1995) Effect of high-affinity anti-Pseudomonas aeruginosa lipopolysaccharide antibodies induced by immunization on the rate of Pseudomonas aeruginosa infection in patients with cystic fibrosis. J Pediatr 127(5):711–717
Hemachandra S, Kamboj K, Copfer J, Pier G, Green LL, Schreiber JR (2001) Human monoclonal antibodies against Pseudomonas aeruginosa lipopolysaccharide derived from transgenic mice containing megabase human immunoglobulin loci are opsonic and protective against fatal pseudomonas sepsis. Infect Immun 69(4):2223–2229
Horn MP, Zuercher AW, Imboden MA, Rudolf MP, Lazar H, Wu H, Hoiby N, Fas SC, Lang AB (2010) Preclinical in vitro and in vivo characterization of the fully human monoclonal IgM antibody KBPA101 specific for Pseudomonas aeruginosa serotype IATS-O11. Antimicrob Agents Chemother 54(6):2338–2344
Secher T, Fauconnier L, Szade A, Rutschi O, Fas SC, Ryffel B, Rudolf MP (2011) Anti-Pseudomonas aeruginosa serotype O11 LPS immunoglobulin M monoclonal antibody panobacumab (KBPA101) confers protection in a murine model of acute lung infection. J Antimicrob Chemother 66(5):1100–1109
Hostacká A, Majtán V (1997) Serotyping and virulence factors of Pseudomonas aeruginosa clinical isolates. Acta Microbiol Immunol Hung 44(2):141–146
Faure K, Shimabukuro D, Ajayi T, Allmond LR, Sawa T, Wiener-Kronish JP (2003) O-antigen serotypes and type III secretory toxins in clinical isolates of Pseudomonas aeruginosa. J Clin Microbiol 41(5):2158–2160
Fonseca AP, Correia P, Sousa JC, Tenreiro R (2007) Association patterns of Pseudomonas aeruginosa clinical isolates as revealed by virulence traits, antibiotic resistance, serotype and genotype. FEMS Immunol Med Microbiol 51(3):505–516
Le Berre R, Nguyen S, Nowak E, Kipnis E, Pierre M, Quenee L, Ader F, Lancel S, Courcol R, Guery BP, Faure K; Pyopneumagen Group (2011) Relative contribution of three main virulence factors in Pseudomonas aeruginosa pneumonia. Crit Care Med 39(9):2113–2120
Lu Q, Eggimann P, Luyt CE, Wolff M, Tamm M, François B, Mercier E, Garbino J, Laterre PF, Koch H, Gafner V, Rudolf MP, Mus E, Perez A, Lazar H, Chastre J, Rouby JJ (2014) Pseudomonas aeruginosa serotypes in nosocomial pneumonia: prevalence and clinical outcomes. Crit Care 18(1):R17
Lu Q, Rouby JJ, Laterre PF, Eggimann P, Dugard A, Giamarellos-Bourboulis EJ, Mercier E, Garbino J, Luyt CE, Chastre J, Georgescu-Kyburz V, Rudolf MP, Gafner V, Lazar H, Koch H, Perez A, Krämer SD, Tamm M (2011) Pharmacokinetics and safety of panobacumab: specific adjunctive immunotherapy in critical patients with nosocomial Pseudomonas aeruginosa O11 pneumonia. J Antimicrob Chemother 66(5):1110–1116
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13(10):818–829
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22(7):707–710
Luna CM, Blanzaco D, Niederman MS, Matarucco W, Baredes NC, Desmery P, Palizas F, Menga G, Rios F, Apezteguia C (2003) Resolution of ventilator-associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit Care Med 31(3):676–682
Acknowledgments
We thank Holger Koch, Verena Gafner, and Michael P. Rudolf from Kenta Biotech, Schlieren, Switzerland for the development of panobacumab. Now ARIDIS Pharmaceuticals, San Jose, USA.
Author contributions
Y-AQ, J-LP, PE, and J-PR acquired patient data and prepared the manuscript.
J-PR performed the statistical analysis.
QL, J-JR, JC, MW, MT, EM, JG, BF, and P-FL acquired patient data.
AP, HL, and EM designed the study, and collected and analyzed the data from the original study.
Conflict of interest
The following authors declare that they have no competing interests: Jorge Garbino, Qin Lu, Jean-Jacques Rouby, Emmanuelle Mercier, Michael Tamm, Michel Wolff.
The following authors were employees of Kenta Biotech at the time of the trial and owned stocks of Kenta Biotech: Holger Koch, Hedvika Lazar, Erkan Mus, Michael P. Rudolf.
Jean Chastre has received consulting and/or lecture fees from Kenta Biotech, Pfizer, Brahms, Astellas, KaloBios, Sanofi, Nektar–Bayer, and Glaxo–Smith–Kline.
Philippe Eggimann has received consulting and/or lecture fees from Kenta Biotech, Pfizer, Astellas, KaloBios, and MSD. He was involved as a consultant for other Kenta Biotech projects.
Bruno François has received consulting and/or lecture fees in the last 3 years from Kenta Biotech, Sanofi, Talecris, GSK, and MedImmune.
Verena Gafner is a former employee of Kenta Biotech.
Pierre-Francois Laterre is a consultant at Kenta Biotech, Agennix, and AstraZeneca.
Antonio Perez is external Chief Medical Officer for Kenta Biotech. Now ARIDIS, Clinical Development Advisor.
Funding
The phase IIa study, but not this independent post-hoc analysis, was sponsored by Kenta Biotech AG. Now ARIDIS Pharmaceuticals, San Jose, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Que, YA., Lazar, H., Wolff, M. et al. Assessment of panobacumab as adjunctive immunotherapy for the treatment of nosocomial Pseudomonas aeruginosa pneumonia. Eur J Clin Microbiol Infect Dis 33, 1861–1867 (2014). https://doi.org/10.1007/s10096-014-2156-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-014-2156-1