Abstract
Chronic inflammation is a well-known risk factor for type 2 diabetes mellitus (T2DM). The influence of chronic osteomyelitis (COM), an inflammatory disease, on the risk of developing T2DM remains unknown. This study investigated the risk of developing T2DM among COM patients. Using a retrospective cohort study, we identified 20,641 patients with COM and 82,564 age- and sex-matched controls for comparison from the Taiwan National Health Insurance Database (NHIRD) from 1997 to 2010. We followed up the COM cohort and the comparison cohort to compare the incidences of diabetes (ICD-9-CM code 250) until the end of 2010 or until the patients were censored because of death or withdrawal from the insurance program. The diabetes risk was analyzed using the Cox proportional hazards regression model. The incidence of T2DM was 1.6-fold higher in the group of COM patients than in the comparison group (29.1 vs. 18.2 per 10,000 person-years). The COM patients exhibited a higher diabetes risk [adjusted hazard ratio (aHR) = 1.64, 95 % confidence interval (CI) = 1.44–1.87] after controlling for the baseline and comorbidities. Younger and higher income patients exhibited a higher COM-to-reference incidence rate ratio (IRR) for T2DM compared with that of their counterparts. We also observed an increased risk of T2DM in COM patients with comorbidities (aHR = 1.70, 95 % CI = 1.47–1.96) compared with that of their non-COM counterparts. This is the first study to report the association between COM and an increased risk of developing T2DM, particularly among younger and higher income patients.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM), characterized by hyperglycemia and dyslipidemia, is a metabolic disorder caused by imbalance among β-cell function, physical inactivity, obesity, chronic fuel surfeit status, and insulin resistance [1]. T2DM may lead to a substantial health burden on people and direct and indirect burdens on the healthcare system and society [2].
The prevalence of diabetes has increased substantially in Asian countries in recent years [3]. This increase has been attributed to the increase in central obesity, physical inactivity, the aging population, and the prevalence of Western diets in Asian populations [3–5]. Although the metabolic manifestations in both types of diabetes are similar, T2DM is more heterogeneous than type 1 diabetes, and involves additional aspects, including genetic factors, epigenetic effects, early-life environment, lifestyle, nutrition imbalance, socioeconomic status, a deregulated neurohumoral network, and insulin resistance [6–9]. A previous study documented that adipocyte dysfunction may increase the concentrations of inflammatory cytokines and aggravate insulin resistance in muscle [10]. However, the underlying mechanism and complex interaction between T2DM and inflammation, particularly the chronic ones (e.g., adipocyte inflammation, periodontitis, and hepatitis C infection), remain unclear [10–12].
Chronic osteomyelitis (COM), a well-known chronic infection–inflammation status that is resistant to treatment and prone to relapse, is a common complication of T2DM [13]. After conducting a literature review regarding the relationship between T2DM and COM, we observed that most previous studies have focused on the risk of COM among patients with T2DM [13–16], whereas few have addressed the possibility that COM might be a risk factor for T2DM. Using a large cohort of 23 million patients identified from the Taiwan National Health Insurance Database (NHIRD), we assessed the risk of newly developing T2DM among patients with COM.
Materials and methods
Data sources
We used reimbursement claims data from the Taiwan National Health Insurance (NHI) program launched in March 1995. The dataset is managed by the National Health Research Institutes (NHRI) and the details of the NHI program have been described elsewhere [17]. We used a subset of the NHIRD containing healthcare data, including files on inpatient claims and a registry of beneficiaries. The International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) was used to identify diseases. The high accuracy and validity of the T2DM and COM diagnosis used in this study based on the ICD-9 codes applied in the NHIRD were previously confirmed [18–20]. Taiwan launched the NHI program in 1995, which is operated by a single buyer, the government. All insurance claims are scrutinized by medical reimbursement specialists and through peer review. The diagnoses of T2DM and COM were based on the ICD-9 codes determined by clinical physicians. Therefore, the diagnoses and codes for T2DM and COM should be accurate and reliable.
Study patients
The COM cohort included patients with newly diagnosed COM (ICD-9-CM code 730.1) from 1997 to 2010. For each COM patient, four people without a medical history of COM and diabetes who were frequency-matched according to sex and age (each 5-year span) in the same period were randomly selected as the comparison cohort. The index date for the COM cohort was defined as the date of diagnosis. The index date for the comparison cohort was defined as the middle date of the same index month used for their matched COM patients. Patients with a medical history of diabetes who were diagnosed before the index date, younger than 20 years old, or were missing information on age or sex were excluded.
Outcome measures
We followed up the COM cohort and the comparison cohort to compare the incidences of diabetes (ICD-9-CM code 250) until the end of 2010 or until the patient was censored because of death or withdrawal from the insurance program. Sociodemographic characteristics including sex, age, and income of the two cohorts were assessed. The baseline comorbidities included hypertension (ICD-9-CM codes 401–405), hyperlipidemia (ICD-9-CM code 272), and chronic kidney disease (CKD) (ICD-9-CM code 585).
Statistical analysis
Differences in sociodemographic factors and comorbidities between the COM and the comparison cohorts were examined using the Chi-square test for category variables and the t-test for continuous variables. The incidence–density rates according to demographic status and comorbidity were calculated for both cohorts. The incidence rate ratio (IRR) with a 95 % confidence interval (CI) was calculated using the Poisson regression model. Multivariate Cox proportional hazard models, adjusted for potential confounding factors, were used to assess the risk of developing diabetes associated with COM. To assess the difference in the diabetes-free rates between the two cohorts, the Kaplan–Meier analysis and log-rank test were applied. The statistical significance level was set at a two-tailed probability value of p < 0.05. All analyses were performed using SAS software (SAS System for Windows, version 9.1) and the Kaplan–Meier survival curve was plotted using R software (R Foundation for Statistical Computing, Vienna, Austria).
Ethics and consent
Personal information was de-identified before the release of the NHIRD data; therefore, this study was exempt from approval by the Institutional Review Board.
Results
This study consisted of 20,641 patients in the COM cohort and 82,564 people in the comparison cohort. Both cohorts exhibited similar sex and age distributions. Comorbidities including hypertension, hyperlipidemia, and CKD were more prevalent in the COM cohort than in the comparison cohort (p < 0.0001) (Table 1).
The COM cohort had a higher incidence rate of diabetes than the comparison cohort (29.1 vs. 18.2 per 10,000 person-years; IRR = 160, 95 % CI = 1.43–1.86), with an adjusted hazard ratio (aHR) of 1.64 (95 % CI = 1.44–1.87) (Table 2). The COM-to-reference IRR was higher in men (IRR = 1.67, 95 % CI = 1.58–1.77) than in women (IRR = 1.45, 95 % CI = 1.33–1.58). The age-specific IRR increased with age and was the highest in the subgroup aged 20–34 years (IRR = 5.90, 95 % CI = 5.26–6.62), with an aHR of 4.74 (95 % CI = 2.37–9.49). The aHR of diabetes was the highest in the subgroup with a monthly income of over 22,800 New Taiwan dollars (NT$) (aHR = 3.37, 95 % CI = 2.04–5.55).
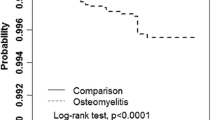
The incidence rate of diabetes was higher in the subgroups with hypertension, hyperlipidemia, or CKD compared with that of the non-comorbid counterparts. The COM-to-reference IRR was 1.58 (95 % CI = 1.06–1.32) and 1.18 (95 % CI = 1.50–1.67) for the groups without and with hypertension, respectively. The significant COM-to-reference IRR was also observed in the non-hyperlipidemia and non-CKD groups. Moreover, the aHRs indicated that COM was associated with an increased risk of diabetes in patients without hypertension (aHR = 1.70, 95 % CI = 1.47–1.96), hyperlipidemia (aHR = 1.66, CI = 1.45–1.89), or CKD (aHR = 1.65, 95 % CI = 1.45–1.87) (Table 3). The Kaplan–Meier survival analysis revealed that the diabetes-free rate was 1.18 % lower in the COM cohort than in the comparison cohort (log-rank p < 0.0001) (Fig. 1).
Discussion
This study yielded several intriguing results. First, this study is the first population-based cohort study to report a significantly increased T2DM risk associated with COM. Second, the association between COM and the increased risk of T2DM was stronger in the wealthier subgroup and the younger subgroup.
The biological mechanisms of the relationship between COM and the risk of T2DM remain unclear. Previous studies have demonstrated that increasing levels of proinflammatory cytokines that block downstream insulin signaling and interrupt insulin action, such as TNF-α, interleukin-β, and interleukin-6, contribute to preclinical stages of T2DM [21, 22]. In COM patients, these proinflammatory cytokines were observed to increase markedly in the bone compartment [23, 24]. Therefore, we suspected that localized proinflammatory cytokines might exert system effects on whole-body insulin resistance. In addition to the proposed effect of proinflammatory cytokines, other possible explanations exist for the association between COM and the risk of T2DM. First, patients with COM might make frequent medical visits and have routine check-ups, including check-ups on biochemical profiles and blood glucose levels, which may increase the likelihood of detecting T2DM. Second, patients with COM are typically immobile because of pain or difficulty in engaging in physical activity [25]. Because physical activity prevents the occurrence of T2DM [26], the immobile status of COM patients might increase the risks of obesity, metabolic syndrome, and T2DM. Furthermore, the genetic predisposition of these two diseases to COM is linked with IL-1α, IL-4, and IL-6 polymorphism [27]. A Finnish diabetes prevention study reported that promoter polymorphisms of TNF-α and IL-6 could predict the risk of T2DM [28]. The association between COM and the risk of T2DM might also result from the genetic predisposition to both COM and T2DM of our study cohorts. Further studies on genetic analysis and the role of physical activity are recommended in order to elucidate the association.
The present results indicated that, in the subgroup with higher income, COM patients had a higher risk of developing T2DM compared with that of the non-COM counterparts, which is intriguing because T2DM has been reported to be more prevalent among those with a lower socioeconomic status [29–31]. Socioeconomic disparities are associated with insulin resistance and altered glucose metabolism [32, 33]. One possible explanation for our finding is that the effects of insulin resistance produced by COM are more easily observed in wealthier people who are assumed to have fewer alterations in glucose metabolism. Future studies are warranted for further investigation of this discrepancy.
In addition, we observed that, among young people aged less than 55 years, COM patients had a higher risk of T2DM compared with that of the non-COM people, which is remarkable because T2DM is a well-known age-related disease [34]. Chronic low-grade inflammation is a converging process linking both normal aging and age-related diseases, including T2DM [35–37]. However, the present study reported that younger patients had a significantly higher COM-to-reference hazard ratio of diabetes mellitus than the elderly subgroup. The underlying mechanism requires further investigation. We suggest that aging might mask the effects of the parallel, pathogenic, inflammation-related process of COM in the development of T2DM.
This study has several strengths. It is the first population-based study in which the risk of T2DM was compared between patients with and without COM. Patients with COM and the age- and sex-matched controls were identified from a dataset of 23 million enrollees in a national insurance program comprising over 98 % of the entire population in Taiwan. Insurance claims of hospitalization and consecutive care of COM ensure the diagnosis of COM. The Taiwan NHI program has a strict auditing system to prevent fraudulent healthcare claims, thereby ensuring the reliability of COM diagnosis based on insurance claims. This large population-based database containing comprehensive electronic medical records provided complete information on the incidence of T2DM and associated comorbidities, including hypertension, hyperlipidemia, and CKD.
However, several limitations should be addressed. We did not obtain precise information on patients’ body weight, body mass index, and fat distribution. Information on physical activity was also unavailable. Additionally, we could not rule out the possibility that the immobilization exhibited by a proportion of COM patents might predispose COM patients to a higher risk of T2DM. Consequently, the T2DM risk assessed in this study might be overestimated. Although our data indicated that comorbidities were more prevalent among patients with COM compared with the comparison cohort, the results suggested a significant association between COM and T2DM after we controlled for comorbidities and sociodemographic characteristics. Future studies are required in order to investigate the underlying mechanisms and confirm the causal relationship between COM and T2DM.
In conclusion, the present study reported a significantly increased risk of developing T2DM in COM patients. We also observed an increased risk of T2DM in young and wealthy COM patients. The findings of this study emphasize the importance of detecting T2DM in COM patients because medical delays and negligence may reduce the quality of medical care and increase healthcare costs. Therefore, in addition to providing adequate antimicrobial treatment and surgical intervention for COM patients, physicians should be aware of the possibility of developing T2DM in these patients, particularly in those who are young and economically advantaged.
References
Nolan CJ, Damm P, Prentki M (2011) Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 378:169–181
Crandall JP, Knowler WC, Kahn SE et al (2008) The prevention of type 2 diabetes. Nat Clin Pract Endocrinol Metab 4:382–393
Chan JC, Malik V, Jia W et al (2009) Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 301:2129–2140
Misra A, Khurana L (2008) Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab 93(11 Suppl 1):S9–S30
Liu KH, Chan YL, Chan WB et al (2006) Mesenteric fat thickness is an independent determinant of metabolic syndrome and identifies subjects with increased carotid intima-media thickness. Diabetes Care 29:379–384
Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research et al (2007) Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316:1331–1336
Pinney SE, Simmons RA (2010) Epigenetic mechanisms in the development of type 2 diabetes. Trends Endocrinol Metab 21:223–229
Malik VS, Popkin BM, Bray GA et al (2010) Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 33:2477–2483
Popkin BM, Horton S, Kim S et al (2001) Trends in diet, nutritional status, and diet-related noncommunicable diseases in China and India: the economic costs of the nutrition transition. Nutr Rev 59:379–390
Guilherme A, Virbasius JV, Puri V et al (2008) Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9:367–377
Ide R, Hoshuyama T, Wilson D et al (2011) Periodontal disease and incident diabetes: a seven-year study. J Dent Res 90:41–46
Basaranoglu M, Basaranoglu G (2011) Pathophysiology of insulin resistance and steatosis in patients with chronic viral hepatitis. World J Gastroenterol 17:4055–4062
Hatzenbuehler J, Pulling TJ (2011) Diagnosis and management of osteomyelitis. Am Fam Physician 84:1027–1033
Game F (2010) Management of osteomyelitis of the foot in diabetes mellitus. Nat Rev Endocrinol 6:43–47
Berendt AR, Peters EJ, Bakker K et al (2008) Diabetic foot osteomyelitis: a progress report on diagnosis and a systematic review of treatment. Diabetes Metab Res Rev 24(Suppl 1):S145–S161
Senneville E, Lombart A, Beltrand E et al (2008) Outcome of diabetic foot osteomyelitis treated nonsurgically: a retrospective cohort study. Diabetes Care 31:637–642
Kreng VB, Yang CT (2011) The equality of resource allocation in health care under the National Health Insurance System in Taiwan. Health Policy 100:203–210
Chung WS, Peng CL, Lin CL et al (2013) Rheumatoid arthritis increases the risk of deep vein thrombosis and pulmonary thromboembolism: a nationwide cohort study. Ann Rheum Dis. doi:10.1136/annrheumdis-2013-203380
Chung WS, Lin CL, Ho FM et al (2014) Asthma increases pulmonary thromboembolism risk: a nationwide population cohort study. Eur Respir J 43:801–807
Cheng CL, Kao YH, Lin SJ et al (2011) Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 20:236–242
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444:860–867
Medzhitov R, Janeway C Jr (2000) Innate immunity. N Engl J Med 343:338–344
Fullilove S, Jellis J, Hughes SP et al (2000) Local and systemic concentrations of tumour necrosis factor-alpha, interleukin-6 and interleukin-8 in bacterial osteomyelitis. Trans R Soc Trop Med Hyg 94:221–224
Biffl WL, Moore EE, Moore FA et al (1996) Interleukin-6 delays neutrophil apoptosis via a mechanism involving platelet-activating factor. J Trauma 40:575–578
Gonzalez BE, Teruya J, Mahoney DH Jr et al (2006) Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics 117:1673–1679
Helmrich SP, Ragland DR, Leung RW et al (1991) Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med 325:147–152
Tsezou A, Poultsides L, Kostopoulou F et al (2008) Influence of interleukin 1alpha (IL-1alpha), IL-4, and IL-6 polymorphisms on genetic susceptibility to chronic osteomyelitis. Clin Vaccine Immunol 15:1888–1890
Kubaszek A, Pihlajamäki J, Komarovski V et al (2003) Promoter polymorphisms of the TNF-alpha (G-308A) and IL-6 (C-174G) genes predict the conversion from impaired glucose tolerance to type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes 52:1872–1876
Rabi DM, Edwards AL, Southern DA et al (2006) Association of socio-economic status with diabetes prevalence and utilization of diabetes care services. BMC Health Serv Res 6:124
Robbins JM, Vaccarino V, Zhang H et al (2001) Socioeconomic status and type 2 diabetes in African American and non-Hispanic white women and men: evidence from the Third National Health and Nutrition Examination Survey. Am J Public Health 91:76–83
Agardh E, Allebeck P, Hallqvist J et al (2011) Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol 40:804–818
McEwen BS, Gianaros PJ (2010) Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci 1186:190–222
Adler NE, Boyce T, Chesney MA et al (1994) Socioeconomic status and health. The challenge of the gradient. Am Psychol 49:15–24
Chung HY, Cesari M, Anton S et al (2009) Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 8:18–30
Chung HY, Sung B, Jung KJ et al (2006) The molecular inflammatory process in aging. Antioxid Redox Signal 8:572–581
Vasto S, Candore G, Balistreri CR et al (2007) Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev 128:83–91
Lorenzo C, Okoloise M, Williams K et al (2003) The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care 26:3153–3159
Acknowledgments
This work was supported by study projects in China Medical University (CMU102-BC-2) the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (DOH102-TD-B-111-004), the Taiwan Ministry of Health and Welfare Cancer Research Center for Excellence (MOHW103-TD-B-111-03), and the International Research-Intensive Centers of Excellence in Taiwan (NSC101-2911-I-002-303). The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation. No additional external funding was received for this study. All authors state that they have no conflicts of interest.
Author contributions
All the authors have contributed substantially and are in agreement on the manuscript content. Conception/design: Shih-Yi Lin, Yen-Jung Chang, Chia-Hung Kao. Provision of study materials: Chia-Hung Kao. Collection and/or assembly of data: all authors. Data analysis and interpretation: Shih-Yi Lin, Cheng-Li Lin, Yen-Jung Chang, Chia-Hung Kao. Manuscript writing: all authors. Final approval of manuscript: all authors.
Conflict of interest
All authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, SY., Lin, CL., Tseng, CH. et al. The association between chronic osteomyelitis and increased risk of diabetes mellitus: a population-based cohort study. Eur J Clin Microbiol Infect Dis 33, 1647–1652 (2014). https://doi.org/10.1007/s10096-014-2126-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-014-2126-7