Abstract
The prospective case series study presented here was conducted to assess the outcome of patients with infections caused by polymyxin-only-susceptible (POS) gram-negative bacteria managed with intravenous colistin. Between July 2003 and April 2005 a total of 27 patients were infected with a POS gram-negative bacterium and received intravenous colistin at a dose of 2 million international units (MIU) (160 mg or 66.7 mg colistin base) every 8 h for a mean (±SD) duration of 13.9 (±7.5) days. Nine patients had ventilator-associated pneumonia and received, in addition to the intravenous colistin therapy, 1 MIU (80 mg or 33.3 mg colistin base) aerosolized colistin every 12 h for a mean (±SD) duration of 13 (±6.5) days. The predominant pathogens were Pseudomonas aeruginosa (n=17) and Acinetobacter baumannii (n=12); in two patients both pathogens were isolated from one clinical specimen. In-hospital mortality and clinical response were 15% and 85%, respectively. Colistin-associated nephrotoxicity was observed in two of the 27 patients. POS gram-negative pathogens represent a major threat for hospitalized patients. Colistin appears to be an effective and safe treatment, even in patients with severe underlying diseases.
Similar content being viewed by others
Introduction
The medical community has been increasingly challenged by the emergence of infections caused by multidrug-resistant gram-negative microorganisms resistant to various classes of antimicrobial agents [1]. Several therapeutic strategies, including the use of antimicrobial combinations of intravenous (iv) polymyxins with other antimicrobial agents possessing a spectrum of activity against gram-negative bacteria, have already been evaluated for managing these infections [2–4].
Data from recent studies suggest that iv colistin or polymyxin B may be valuable therapeutic options when confronting these types of infection [5–11]. However, most of these studies evaluated the use of polymyxins in patients with a mixture of infections caused by multidrug-resistant or polymyxin-only-susceptible (POS) gram-negative bacteria without specifically reporting separate data regarding outcome in these two important subsets of patients [2, 4, 7–9]. As a result, definitive conclusions about the effectiveness of iv colistin for the treatment of infections caused by gram-negative pathogens susceptible only to this class of antibiotics cannot be made. To clarify this issue, we performed a prospective case series study to examine the value of iv colistin as a possible therapeutic option in patients with infections caused by POS gram-negative bacteria.
Patients and methods
A prospective, observational, case series study was conducted at a 650-bed university hospital in Crete, Greece, from July 2003 to April 2005. During this period, patients in whose clinical specimens a POS gram-negative microorganism was identified by the hospital’s laboratory and who received iv colistin were included in the study. Patients who received treatment with iv colistin for less than 24 h or who received antimicrobial regimens without colistin were excluded from the analysis. The hospital’s Institutional Review Board approved the study.
Using a detailed case report form, we recorded data from the medical records of the patients. The microbiological information collected included the causative organisms and their in vitro susceptibilities. In addition, data from laboratory tests such as renal function tests performed on the first and last day of colistin treatment were also collected. Two researchers independently determined the type of infection, the causative pathogen, and the clinical outcome.
Susceptibility testing of gram-negative microorganisms was performed using an automated broth microdilution method (Organon Teknika Corp, Durham, NC, USA). The breakpoints used were those defined by the Clinical and Laboratory Standards Institute (CLSI) [12]. Susceptibility to colistin was also tested using the E test methodology (sensitive, 2 mg/l or less; resistant, 4 mg/l or more) and the disk diffusion method with a 10 μg colistin sulfate disk. Isolates were considered susceptible if the inhibition zone was ≥11 mm and resistant if the inhibition zone was ≤8 mm. An isolate was defined as POS if it was susceptible to polymyxins but resistant to agents from the six antipseudomonal antimicrobial classes, i.e. antipseudomonal penicillins, cephalosporins, carbapenems, monobactams, quinolones, and aminoglycosides. In-hospital mortality, clinical outcome of infection, and development of nephrotoxicity during treatment with iv colistin were the main outcomes investigated in this study. Infections were defined based on the guidelines from the Centers for Disease Control and Prevention [13]. The clinical outcome of infection and nephrotoxicity were based on previously established definitions [2].
The comparison of means between dependent observations (such as serum creatinine concentration at the start and end of iv colistin administration in the analyzed patients) was performed using the Wilcoxon test and between independent observations using the Mann–Whitney test.
Results and discussion
During the study period from July 2003 to April 2005, a total of 36 patients were hospitalized with infections caused by POS gram-negative bacteria. Nine patients were excluded from the study because they did not receive iv colistin treatment. In-hospital mortality was 33% (3/9 patients) in this group of patients. Thus, 27 patients were analyzed further based on the study design.
Table 1 summarizes various demographic and clinical characteristics of the study cohort. All of the patients studied received treatment with iv colistin initially at a dose of 2 MIU (160 mg colistimethate sodium or 66.7 mg colistin base) q8h. One milligram of the colistin formulation used is approximately equal to 12,500 IU colistimethate sodium (CMS) (Norma®, Athens, Greece). Dosages were adjusted in the presence of renal dysfunction [2]. In nine patients with VAP, aerosolized colistin was administered in addition to iv colistin therapy at a dose of 1 MIU (80 mg or 33.3 mg colistin base) q12h. The mean (±SD)/median durations of intravenous and aerosolized colistin treatment were 13.9 (±7.5)/13 days (range 2–40 days) and 13 (±6.5)/14.2 days (range, 2–21 days), respectively.
Polymicrobial infections were observed in ten patients. Nine patients were infected by two different gram-negative strains, and in one patient three gram-negative isolates were grown from culture specimens. Overall, 38 different strains of POS gram-negative microorganisms were isolated from the 27 patients evaluated. The most common pathogen was P. aeruginosa (17 isolates) followed by A. baumannii (12 isolates), Klebsiella pneumonia (5 isolates) and Escherichia coli (4 isolates). Gram-positive cocci were isolated from 14 of the 27 patients. Enterococcus faecalis grew in nine patients, Staphylococcus aureus in three patients, Enterococcus faecium in one patient and coagulase-negative Staphylococcus in one patient. Candida species were isolated from five of the 27 evaluable patients.
Intravenous colistin monotherapy was administered to five of the 27 evaluable patients in order to manage their infections due to POS microorganisms. In 19 patients an additional antimicrobial agent with activity against gram-negative bacteria was given intravenously during the course of colistin treatment. In addition, three of the 27 patients received two antibiotics concurrently with iv colistin. Specifically, quinolones were given to ten patients, piperacillin/tazobactam to five patients, meropenem to three patients, ampicillin, ceftazidime, and ampicillin/sulbactam to two patients each, and ceftriaxone to one patient. Antimicrobial agents with activity against gram-positive cocci were also given to 14 of the 27 patients, including vancomycin in ten patients, teicoplanin in two patients, and linezolid in two patients.
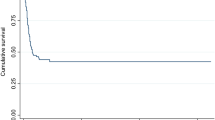
The all-cause in-hospital mortality in this cohort of 27 patients was 15% (4/27 patients). Two of the four patients who died had VAP and two had a urinary tract infection. In all cases death was probably related to the infection. In three of them, the causative pathogen isolated was a P. aeruginosa strain and the other patient had a polymicrobial infection. None of the patients who died had shown clinical evidence of response during colistin therapy. In the subgroup of patients who received aerosolized colistin concomitantly, mortality was 22%.
Table 2 shows the clinical response observed in this patient population with respect to the site of infection. Clinical response (i.e. cure or improvement) was observed in 23 of the 27 patients; 17 were defined as cured and six as improved. Failure of the colistin treatment administered to control the infection was observed in four of the 27 patients. The mean (±SD) length of hospital stay was 48 (±43) days (range 8–177) and the mean (±SD) duration of ICU stay was 18 (±17) days (range 3–62). Nephrotoxicity was observed in two of the 27 evaluable patients.
The mean (±SD)/median serum creatinine concentration observed at the initiation of iv colistin therapy in the studied population was 1.0 (±0.67)/0.9 mg/dl (range 0.3–3.2). At the end of iv colistin therapy, the mean (±SD) creatinine level had increased to 1.2 (±1.1) mg/dl (range 0.3–4.3). This slight increase of serum creatinine levels by an average of 0.2 mg/dl at the completion of colistin treatment was not statistically significant (p=0.4). Blood urea levels were not substantially different at the start and end of iv colistin administration; mean (±SD) baseline levels were 69.1 (±44.3) mg/dl (range 11–170) and end of treatment levels were 64.6 (±45.8) mg/dl (range 6–169). The two patients who developed nephrotoxicity during colistin treatment received 6 MIU for 10 and 12 days, respectively. Serum creatinine had increased from 0.9 mg/dl to 1.9 mg/dl at the end of treatment in one patient, while the other received hemodialysis treatment (baseline and end-of-treatment serum creatinine levels: 3.2 mg/dl and 4.3 mg/dl, respectively). In both patients renal function returned to normal values after cessation of colistin treatment. In the subgroup of four patients who experienced a treatment failure, serum baseline and end-of-treatment creatinine levels were 0.6 mg/dl, 0.9 mg/dl, 0.9 mg/dl, 0.7 mg/dl and 1.0 mg/dl, 0.9 mg/dl, 1.9 mg/dl, 1.3 mg/dl, respectively. No statistically significant changes were noted between baseline and end-of-treatment levels of serum glutamic–pyruvic transaminase, serum glutamic–oxaloacetic transaminase, alkaline phosphatase, and gamma-glutamyl transpeptidase in the study population.
The main findings of this prospective case series study of patients who received colistin therapy for infections caused by POS gram-negative bacteria include a survival rate of 85% (23/27 patients) and a clinical response rate of 85% (23/27 patients). In addition, nephrotoxicity related to colistin therapy was observed in 7% of the study population (2/27 patients). Our data support those of previously published studies with respect to the effectiveness and safety of iv colistin for treating patients with infections caused by POS gram-negative bacteria [10, 11]. However, we should acknowledge that our study has several limitations. In addition to the lack of a control group, the number of patients we included was relatively small, so strong conclusive statements can not be made, and since iv colistin was used in combination with other antimicrobial agents, it is difficult to clarify whether or not the promising effects we observed could also have been achieved with use of any antibiotic alone.
It is noteworthy that one vial of the colistin formulation manufactured in Europe (Alpharma, Copenhagen, Denmark) contains 80 mg (1 MIU) of CMS, while a vial of the colistin formulation manufactured in the USA (Parkedale Pharmaceuticals, Rochester, MI, USA) contains approximately 150 mg of colistin base activity. One milligram of colistin base is contained in 2.4 mg of CMS and equals 30,000 IU. Thus, caution is needed when dosing different formulations of colistin in various parts of the world [14]. In conclusion, our study provides additional information suggesting that colistin may be a rational treatment for managing patients infected with POS bacteria. This is of particular importance in an era when the emergence of infections due to these pathogens represents a major health problem, especially due to the lack of established alternative therapeutic options.
References
Navon-Venezia S, Ben Ami R, Carmeli Y (2005) Update on Pseudomonas aeruginosa and Acinetobacter baumannii infections in the healthcare setting. Curr Opin Infect Dis 18:306–313
Kasiakou SK, Michalopoulos A, Soteriades ES, Samonis G, Sermaides GJ, Falagas ME (2005) Combination therapy with intravenous colistin for management of infections due to multidrug-resistant gram-negative bacteria in patients without cystic fibrosis. Antimicrob Agents Chemother 49:3136–3146
Levin AS, Barone AA, Penco J, Santos MV, Marinho IS, Arruda EA et al (1999) Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis 28:1008–1011
Markou N, Apostolakos H, Koumoudiou C, Athanasiou M, Koutsoukou A, Alamanos I et al (2003) Intravenous colistin in the treatment of sepsis from multiresistant gram-negative bacilli in critically ill patients. Crit Care 7:R78–R83
Ouderkirk JP, Nord JA, Turett GS, Kislak JW (2003) Polymyxin B nephrotoxicity and efficacy against nosocomial infections caused by multiresistant gram-negative bacteria. Antimicrob Agents Chemother 47:2659–2662
Sobieszczyk ME, Furuya EY, Hay CM, Pancholi P, Della-Latta P, Hammer SM et al (2004) Combination therapy with polymyxin B for the treatment of multidrug-resistant gram-negative respiratory tract infections. J Antimicrob Chemother 54:566–569
Linden PK, Kusne S, Coley K, Fontes P, Kramer DJ, Paterson D (2003) Use of parenteral colistin for the treatment of serious infection due to antimicrobial-resistant Pseudomonas aeruginosa. Clin Infect Dis 37:e154–e160
Berlana D, Llop JM, Fort E, Badia MB, Jodar R (2005) Use of colistin in the treatment of multiple-drug-resistant gram-negative infections. Am J Health-Syst Pharm 62:39–47
Falagas ME, Rizos M, Bliziotis IA, Rellos K, Kasiakou SK, Michalopoulos A (2005) Toxicity after prolonged (more than four weeks) administration of intravenous colistin. BMC Infect Dis 5:1–8
Michalopoulos AS, Tsiodras S, Rellos K, Mentzelopoulos S, Falagas ME (2005) Colistin treatment in patients with ICU-acquired infections caused by multiresistant gram-negative bacteria: the renaissance of an old antibiotic. Clin Microbiol Infect 11:115–121
Reina R, Estenssoro E, Saenz G, Canales HS, Gonzalvo R, Vidal G et al (2005) Safety and efficacy of colistin in Acinetobacter and Pseudomonas infections: a prospective cohort study. Intensive Care Med 31(Suppl 8):1058–1065
Wayne P (2000) Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Approved standards document M7–A5, Fifth edn. National Committee for Clinical Laboratory Standards, Wayne, PA
Gaynes RP, Horan TC (1996) Surveillance of nosocomial infections. Appendix A: CDC definitions of nosocomial infections. In: Mayhall CG (ed) Hospital epidemiology and infection control. Williams & Wilkins, Baltimore, pp 1–14
Falagas ME, Kasiakou SK (2006) Use of international units when dosing colistin will help decrease confusion related to various formulations of the drug around the world. Antimicrob Agents Chemother 50:2274–2275
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Falagas, M.E., Kasiakou, S.K., Kofteridis, D.P. et al. Effectiveness and nephrotoxicity of intravenous colistin for treatment of patients with infections due to polymyxin-only-susceptible (POS) gram-negative bacteria. Eur J Clin Microbiol Infect Dis 25, 596–599 (2006). https://doi.org/10.1007/s10096-006-0191-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-006-0191-2