Abstract
Objective
Our study aimed to investigate the correlations between microstructural changes of cingulum and patients with mild cognitive impairment (MCI) by diffusion kurtosis imaging (DKI) technique.
Method
A total of 104 patients with cerebral small vessel diseases (cSVD) were retrospectively enrolled in this study. According to Montreal Cognitive Assessment Scale (MoCA) scores, these patients were divided into MCI group (n = 59) and non-MCI group (n = 45). The general clinical data was collected and analyzed. The regions of interests (ROIs) were selected for investigation in cingulum. The values of DKI parameters were measured in each ROI and compared between the two groups, the correlations between DKI parameters and MoCA scores were examined.
Results
Compared to non-MCI group, MCI patients had more severe white matter hyperintensities (WMHs) (P = 0.038) and lower MoCA scores (P < 0.01). MCI patients showed significantly decreased fractional anisotropy (FA), axial kurtosis (AK), mean kurtosis (MK), radial kurtosis (RK), and kurtosis fractional anisotropy (KFA) in the left cingulum in the cingulated cortex (CgC) region (all P < 0.0125). In the left CgC region, FA, AK, MK, RK, and KFA were positively correlated with MoCA scores (r = 0.348, 0.409, 0.310, 0.441, 0.422, all P < 0.001). Meanwhile, FA, AK, MK, RK, and KFA were also positively correlated with MoCA scores (r = 0.338, 0.352, 0.289, 0.380, 0.370, all P < 0.001) in the right CgC region.
Conclusion
DKI technique could be used to explore the microstructural changes of cingulum in MCI patients and DKI-derived parameters might be feasible to evaluate MCI patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral small vessel diseases (cSVD) is a disease with a high prevalence related to age; it is mainly including WMHs, recent small subcortical infarct, prominent perivascular spaces (PVS), cerebral microbleeds (CMBs), lacunes, and atrophy [1]. The prevalence of WMHs increased from 50 to 95% at the age of 45–80 years [2]. The prevalence of brain microbleeds is 24%; it gradually increases with age and reaches up to 38.8% in the patients over the age of 80 years [3]. The recent studies based on Chinese populations have shown that lacunar infarction accounts for 38–46% of ischemic stroke [4,5,6].
cSVD is thought to be one of the main causes of MCI. Growing evidence indicates that cerebrovascular pathology is the most important contributor to dementia. However, pure vascular dementia is relatively uncommon, accounting only 10% of all dementia cases, whereas multiple-etiology dementia with a vascular component, most often in combination with Alzheimer’s disease (AD), is more common and accounts for approximately 30 to 40% of all dementia cases [7, 8]. MCI is a common condition encountered by clinicians, the prevalence in persons 60 years and older was estimated between 15 and 20%, and the annual rate in which MCI progresses to dementia varies between 8 and 15% per year [9]. A meta-analysis that assessed the reversion rates from MCI to normal cognition in 25 studies indicated an overall reversion rate of approximately 24% [10]. The most cognitive impairment in the elderly arises from multiple pathologies, of which the vascular component is currently one of the treatable and preventable etiologies. Therefore, early recognition and timely intervention of MCI patients with cSVD may slow the progression to dementia. Several studies have confirmed that cSVD contributes to multiple domains of cognitive impairment [2, 11, 12]. This is thought to be the result of disruption of white matter (WM) tracts. The cingulum bundle is one of the most distinctive WM tracts, which interconnects frontal, parietal, and medial temporal sites, while it is also linking subcortical nuclei to the cingulate gyrus [13]. A recent study by Metoki found that the microstructural abnormality of cingulum is related to mnemonic function in cSVD [14]. This result was consistent with a number of previous studies of correlations between microstructural changes in the cingulum and MCI or AD [15,16,17,18,19,20,21]. However, all the studies mentioned above were analyzed through diffusion tensor imaging (DTI) methods, which cannot accurately describe the complexity of tissue microstructure because of the complexity of tissue structure and cell components. DKI can partially overcome these limitations and DKI parameters have been found to be very sensitive to identify certain changes in many neurological diseases [22, 23]. DKI can detect these microstructural changes even before any imaging findings through conventional imaging, that the reason why it is better than DTI [24]. This study aimed to investigate the correlation between the microstructural changes of cingulum and MCI patients with cSVD by DKI technology, which may provide neuroimaging evidence for the early evaluation of MCI patients.
Material and method
Subjects
We retrospectively collected 104 patients with cSVD from January 2018 to December 2019. The diagnosis was confirmed by conventional head magnetic resonance imaging (MRI) scan and magnetic resonance angiography (MRA) of the head [25]. All the participants underwent a baseline evaluation, including clinical data collection, cognitive function, and neuropsychological assessment. Inclusion criteria were as follows: age of ≥ 50 years, cranial MRI confirmed the presence of cSVD, mainly including lacunar infarcts and/or WMHs [1], complete cognitive and neuropsychological assessment, complete head MRI, including T1-weighted images (T1WI), T2-weighted images (T2WI), fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI), susceptibility-weighted imaging (SWI), DKI, and MRA. Exclusion criteria were as follows: patients with severe neurological diseases, such as AD, Parkinson’s disease or brain trauma; patients with intracranial and external large vascular stenosis; patients with severe medical diseases, such as renal failure, liver diseases, heart diseases, or other systemic diseases; patients with severe mental disease or neuropsychological disorders; patients with low image data quality.
General criteria for MCI
MCI diagnostic criteria were in compliance with the International Working Group on MCI [26]. Firstly, the participants should be judged neither normal nor dementia; Secondly, functional activities of the participants are mainly preserved, or at least that impairment is minimal; Thirdly, the participants should have evidence of cognitive decline, measured either by self and/or informant report in conjunction with deficits on objective cognitive tasks, and/or evidence of decline over time on objective neuropsychological tests.
Cognitive function and neuropsychological assessment
All patients were evaluated by neuropsychological scales at admission. Mini-mental state examination (MMSE) and MoCA scales were applied to assess overall cognitive function and interpret the score according to their social/educational status. MoCA scores were ≤ 13 points for illiterate patients, ≤ 19 points for primary school patients, ≤ 24 points for junior high school and above patients, and the clinical dementia rating (CDR) ≤ 0.5 were considered to be MCI group, otherwise were considered to be non-MCI group [27]. We also assessed the severity of depressive or anxiety disorders by 24 stems of Hamilton Depression Scale (HAMD) and the Hamilton Anxiety Scale (HAMA). Patients with HAMD score < 7 points were considered normal; 7 to 17 points were considered mild depression; 18 to 24 points were considered moderate depression; and > 24 points were considered major depression. Patients with HAMA > 29 were divided into severe anxiety, 22 to 29 have markedly anxious, 15 to 21 have definitely anxious, 8 to 14 were likely to have anxiety, and < 8 was no anxiety symptoms.
Assessment of WMHs
Assessments of WMHs were performed by two experienced radiologists (K. L. and Z. P.). Discrepancies were resolved by consensus. The k statistic of the intra-rater and inter-rater agreement was 0.85 or above, indicating good reliability. WMHs were defined as diffuse or confluent white matter hyperintensities in the periventricular or subcortical white matter observed on T2WI or FLAIR. WMHs were scored by using the Fazekas Scale. A detailed description of these assessments has been previously described. Periventricular white matter hyperintensities (P-WMHs) and deep white matter hyperintensities (D-WMHs) were evaluated separately and summed as Fazekas scores. The degree of WMHs were rated by Fazekas scores (mild: 0 to 2; moderate: 3 to 4; severe: 5 to 6; see Fig. 1) [28].
MRI acquisition
Two radiologists viewed these images. All patients were scanned on a 3 Tesla scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) with a 20-channel phased-array head coil. Foam fillers and earplugs were used to limit head movement and reduce scanner noise. T1WI were acquired using a 3D magnetization-prepared rapid acquisition gradient echo (MPRAGE). The parameters were set as follows: repetition time (TR) = 2300 ms, echo time (TE) = 89 ms, inversion time (TI) = 900 ms, flip angle (FA) = 8°, voxel size = 0.9-mm isotropic, parallel acceleration factor (PAT) = 2, field-of-view (FOV) = 240 × 240 mm2, and acquisition time = 5 min 21 s. Diffusion imaging was performed by using spin-echo plane imaging (SE-EPI) and scanned in two blocks. The sequence parameters of the first block were: TR = 7700 ms, imaging matrix = 74 × 74, TE = 89 ms, FOV = 222 × 222 mm, slice thickness = 3 mm, number of slices = 50, b = 0, 1000, 2000s/mm2, 30 gradient directions, 1 average, PAT = 2, and the acquisition time was 8 min 14 s. The parameters of the second block were the same as those of the first block, except that only b = 0 s/mm2 was used; the average was 9; and the acquisition time was 1 min 34 s. The total time of diffusion scan was 9 min 48 s.
DKI data processing
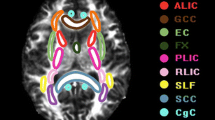
The diffusion images were first transformed to NII file format by using the dcm2nii tool, then, supplied to the diffusional kurtosis estimator (DKE) to generate DKI parameter maps. The T1WI acquired by MPRAGE were supplied to the SPM12 toolbox [29]. The DWI images (b = 0 s/mm2) were strictly aligned with T1WI space, and the transformed matrix was applied to the DKI parameter map. The DKI parameters of ROIs were automatically extracted by using MATLAB (2017a, The MathWorks, Inc., Natick, MA). The parameters of DKI include: MD, AD, RD, FA, MK, AK, RK, and KFA. MK, the most commonly used DKI parameter, means the average of the diffusion kurtosis along all diffusion directions; AK is the kurtosis along the axial direction of the diffusion ellipsoid; RK is the kurtosis along the radial direction of the diffusion ellipsoid; and FA is the most commonly used DTI parameter, which has been a primary imaging metric used in the evaluation of a wide range of neuropathologic processes [30]. The cingulum (CG) in the cingulate gyrus and the hippocampal regions is separated at the axial level of the splenium of the corpus callosum and denoted as CgC and CgH, respectively. CgC and CgH were selected as ROIs according to the ICBM template (see Fig. 2) [31].
Statistical analysis
Statistical analyses were performed using SPSS (version 20.0, IBM Corp., Armonk, NY). The one-sample Kolmogorov–Smirnov test was used to test the normality of the data distribution. Data was expressed as mean ± standard deviation (\(\overline{X }\) ± SD) or median (quartile). The Mann–Whitney U test, the independent t-test, or the χ2 test were applied appropriately for comparison between the two groups. The Bonferroni method was used to correct the P value, and the corrected P value was statistically significant when P < 0.0125 (0. 05/4 = 0.0125). Correlation between the MoCA scale scores and DKI parameters was evaluated with Spearman correlation analysis. A value of P < 0.05 was considered statistically significant.
Results
Comparison of general characteristics
According to the presence or absence of MCI, the 104 patients were divided into MCI group (59 cases) and non-MCI group (45 cases). In MCI group, there were 35 males, the ages ranged from 50 to 88 years old, with a median age of 65 (60, 72) years old, the education period was from 0 to 18 years, and the median duration of education was 8 (7, 10) years. In the non-MCI group, there were 26 males; the ages ranged from 50 to 78 years old, with a median age of 64 (58, 69) years old; the duration of education was from 8 to 11 years, and the median duration of education was 9.5 (8, 11) years. There were no significant differences in age, gender, and years of education between the two groups (P > 0.05).
Patients with MCI had more severe total WMHs (P = 0.038) and had evident decreased cognitive function scores. MMSE and MoCA scores were significantly different between the two groups (both P < 0.001). There were no significant differences in the risk factors of cerebrovascular diseases (such as diabetes mellitus, hypertension, and history of smoking) and the blood test results (such as serum glucose, total cholesterol, and serum homocysteine) between the two groups (see Table 1).
Neuropsychological test scores
The results showed that 27 of the 59 MCI patients had normal MMSE scores; the MoCA scale indicated that in addition to delayed recall impairment, MCI patients mainly combined with damage to visuospatial/executive, language, and abstract functions (see Table 2).
Comparison of DKI parameters in cingulum between the MCI and non-MCI groups
Compared to non-MCI group, the MCI patients showed significantly increased MD and RD (P = 0.03, 0.02 respectively) and significantly decreased FA, AK, MK, RK, and KFA in the left CgC region (P = 0.002, 0.001, 0.001, 0.002, 0.005, respectively). No parameters were found to be significantly different between the two groups in the right CgC region, and both sides of the CgH regions. After correction by Šídák-Bonferroni method, FA, AK, MK, RK, and KFA still remained statistically different in the left CgC region (P = 0.002, 0.001, 0.001, 0.002, 0.005, respectively; see Tables 3–4).
Correlations between DKI parameters and MoCA scale scores
In left CgC region, FA, AK, MK, RK, and KFA were positively correlated with MoCA scores (r = 0.348, 0.409, 0.310, 0.441, 0.422, all P < 0.001), and in the right CgC region, FA, AK, MK, RK, and KFA were also positively correlated with MoCA scores (r = 0.338, 0.352, 0.289, 0.380, 0.370, all P < 0.001). However, the AD, MD, RD of the left and right CgC regions had no correlations with MoCA scores, and the parameters in the CgH regions also had no correlations with MoCA scores (detailed Spearman coefficients are summarized in Table 5).
Discussion
Our study found that compared to non-MCI group, MCI group had more severe WMHs patients. Our study also showed that MoCA scale was more sensitive than MMSE for MCI patients. In addition to delayed recall impairment, MCI patients mainly combined with damage to language, visuospatial/executive and abstract functions. Our study mainly found that MCI patients showed significantly decreased FA, AK, MK, RK, and KFA in the left CgC region. FA, AK, MK, RK, and KFA were significantly positively correlated with MoCA scores in both sides of the CgC regions, while the DKI parameters in the CgH regions had no significant correlations with MoCA scores.
MMSE and MoCA were two widely used cognitive function assessment scales in clinical practice. The meta-analysis found that the sensitivity of MoCA to MCI patients was 80.4%, and the specificity was 81.19%. However, the sensitivity and specificity of MMSE to MCI patients were 66.34% and 72.94%, respectively [32]. Approximately 46% of the MCI patients in our study had normal MMSE scores, suggesting that MMSE had poor sensitivity to MCI patients, which is consistent with the results of previous study [32]. Our study showed that all patients with MCI had delayed recall impairment, and most patients accompanied with multiple cognitive domain impairments. Our results were consistent with those found by Papma et al., which indicated MCI patients with cSVD showed a cognitive profile of prominent memory impairment, dysexecutive functioning, and language problems, when compared with controls [33]. Similar to our results, Boyle et al. also found WMH volume was associated with an increased rate of decline in global cognition, including perceptual speed, working memory, episodic memory, and semantic memory [34]. However, different from our results, previous studies have also shown that patients with vascular brain lesions were impairments mainly in executive function and processing speed [35, 36]. Delayed recall of words in a verbal learning test is a sensitive measure for the diagnosis of amnestic mild cognitive impairment (aMCI), and aMCI is the typical prodromal stage of dementia due to AD [9]. Iadecola et al. found that AD is the leading cause of clinically diagnosed dementia in Western countries; cognitive impairment of vascular etiology is the second most common cause and may be the predominant one in East Asia [35]. As most cognitive impairment in the elderly arises from multiple pathologies, the population enrolled by our study maybe mainly represented with mixed etiology cognitive impairment (mostly vascular + degenerative AD-type).
Our study mainly found that compared to non-MCI group, the MCI patients showed significantly decreased FA, AK, MK, RK, and KFA in the left CgC region. The cingulum was regarded as the core part of the limbic system and also an important part of the cholinergic pathway. However, both the limbic system and the cholinergic pathway are related to cognitive impairment [37, 38]. This tract carries information from the cingulate gyrus to the hippocampus. Our results were consistent with a recent study, which found that individual FA differences in the dorsal/anterior cingulum contribute independently to all executive functions by Bettcher et al. [39]. Kantarci et al. employed ROI approach to explore the contribution of anterior and posterior cingulum FA and MD to executive/attention, language, memory, and visuo-spatial function in a group of 220 cognitive health older adults. They also found FA differences in the anterior cingulum were related to differences in attention/execution and memory, while FA seems to contribute to all four cognitive domains in the post cingulum [40]. Another study used by DTI tractography reconstructed cingulum and found individual FA differences in the anterior and posterior cingulum portion which was correlated with executive function tasks [17]. The reason may be that the anterior cingulum mainly correlated with attention and executive functions, while the parahippocampal cingulum will be more closely linked to learning and episodic memory [13]. The white matter microstructural changes in cingulum may be the reason for the decrease of FA; however, most of the studies on the microstructural changes of cingulum were conducted through the DTI method, and DKI technology was rarely applied [14, 15, 41]. Our previous study found that compared to non-MCI group, MCI patients showed significantly decreased MK in the left hippocampus (P = 0.002) [42]. We also found that MCI patients showed significantly decreased FA, AK, MK, RK, and increased MD and RD in the cingulate gyrus region [43]. Our results showed that kurtosis parameters were suggested to be more sensitive than diffusivity parameters for detecting microstructural changes in the cingulum. The reason for decreased of FA, AK, MK, RK, and KFA may be due to the loss of neuron cell bodies, synapses, and dendrites; the extracellular spaces were increased, and further research is needed to confirm our hypothesis.
Our study also found FA, AK, MK, RK, and KFA were significantly positively correlated with MoCA scores in both sides of the CgC regions. Kantarci et al. also found FA of parahippocampal cingulum was correlated with the severity of AD [44]. Likewise, the relationship between disease severity and cingulum microstructure comes from a study which also found the correlations between AD patients’ MMSE scores and MD in the posterior cingulum [45]. Our previous study also found in the left hippocampal region, FA, RK,MK, and KFA were positively correlated with MoCA scores [42]. Based on above studies, although the specific neurobiological mechanism behind the changes in kurtosis parameters was still unclear, the microstructure changes of DKI parameters may be caused by cerebral atrophy, or may appear before cerebral atrophy. However, the kurtosis and the diffusion parameters complement each other, and DKI technology may provide a certain reference value for the early diagnosis of MCI patients.
In addition, our study also revealed bilateral asymmetry in the microstructural changes of the cingulum in MCI patients. Compared to non-MCI group, the microstructural changes in the left cingulum were more obvious than in the right in MCI patients. Asymmetry plays an essential role in the healthy human brain, and changes in standard asymmetry patterns often mean pathological changes in the brain. Several studies focused on the relationship between abnormalities in brain symmetry and changes in the cognitive abilities. The big majority of these studies were on AD patients and in this population, the left hemisphere has been demonstrated to be significantly more impaired than the right, indicating a faster left hemisphere degeneration in AD[46,47,48]. However, there are only few studies on vascular dementia. A recent study about the changes in gray matter asymmetry and their relationship with cognitive impairment in patients with subcortical ischemic vascular disease (SIVD) found that in the fusiform and parahippocampal gyruses, the SVCI group displayed a dramatic rightward asymmetry [49]. WM asymmetries of the human brain have been well documented using diffusion tensor imaging (DTI) and revealed that cingulum was leftward asymmetry in human brains [47, 50]; our results were consistent with these studies. Our previous study also revealed that the microstructural changes in the left hippocampus were more obvious than in the right in MCI patients [42].
Limitations of the study
Our study also has several limitations. Firstly, we have no normal healthy control patients; both of our two groups were cSVD patients, which may have a certain impact on the results. Secondly, because the MRI scan of the head of the enrolled patients took nearly 1 h, and most AD patients could not tolerate the long-time MRI scan, our study excluded AD patients. However, it has been clearly demonstrated that cSVD contributes to the development of AD and accelerates its progression [35]. So, this may have a certain impact on the results. Thirdly, the sample size of our study is relatively small, which may have contributed to the significant group difference. Furthermore, we did not analyze the volume of the cingulum, and the relationship between the volume of cingulum and the kurtosis diffusion parameters. Finally, due to the small number of MCI patients in our study, we did not further analyze the subtypes of MCI. Although we found that DKI technology has certain imaging diagnostic value in the early diagnosis of MCI patients, further research is still needed.
Conclusion
DKI technology could be applied to observe the microstructural changes of the cingulum in MCI patients with cSVD. Compared to non-MCI group, some DKI parameters of cingulum were significantly different in MCI patents. Furthermore, some DKI parameters showed heterogeneous patterns of correlations with the MoCA scores of MCI patients, which might provide insights into the imaging evaluation of MCI patients.
Data availability
The datasets generated for this study were available on request to the corresponding author.
References
Shi Y, Wardlaw JM (2016) Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc Neurol 1(3):83–92. https://doi.org/10.1136/svn-2016-000035
Wen W, Sachdev PS, Li JJ et al (2009) White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44–48. Hum Brain Mapp 30(4):1155–1167. https://doi.org/10.1002/hbm.20586
Pinter D, Enzinger C, Fazekas F (2015) Cerebral small vessel disease, cognitive reserve and cognitive dysfunction. J Neurol 262(11):2411–2419. https://doi.org/10.1007/s00415-015-7776-6
Tsai CF, Thomas B, Sudlow CL (2013) Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology 81(3):264–272. https://doi.org/10.1212/WNL.0b013e31829bfde3
Fang XH, Wang WH, Zhang XQ et al (2012) Incidence and survival of symptomatic lacunar infarction in a Beijing population: a 6-year prospective study. Eur J Neurol 19(8):1114–1120. https://doi.org/10.1111/j.1468-1331.2012.03709.x
Sun XG, Wang T, Zhang N et al (2015) Incidence and survival of lacunar infarction in a southern Chinese population: a 7-year prospective study. Brain Inj 29(6):739–744. https://doi.org/10.3109/02699052.2015.1004752
Schneider JA, Arvanitakis Z, Bang W et al (2007) Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69(24):2197–2204. https://doi.org/10.1212/01.wnl.0000271090.28148.24
Azarpazhooh MR, Hachinski V (2019) Vascular cognitive impairment: a preventable component of dementia. Handb Clin Neurol 167:377–391. https://doi.org/10.1016/B978-0-12-804766-8.00020-0
Petersen, R.C.,(2016) Mild cognitive impairment. continuum (Minneap Minn). 22(2 Dementia): p. 404–18. https://doi.org/10.1212/CON.0000000000000313.
Malek-Ahmadi M (2016) Reversion from mild cognitive impairment to normal cognition: a meta-analysis. Alzheimer Dis Assoc Disord 30(4):324–330. https://doi.org/10.1097/WAD.0000000000000145
van Norden AG, de Laat KF, Gons RA, et al. (2011) Causes and consequences of cerebral small vessel disease. The RUN DMC study: a prospective cohort study. Study rationale and protocol. BMC Neurol. 11: 29. https://doi.org/10.1186/1471-2377-11-29
Benavente OR, White CL, Pearce L et al (2011) The secondary prevention of small subcortical strokes (SPS3) study. Int J Stroke 6(2):164–175. https://doi.org/10.1111/j.1747-4949.2010.00573.x
Bubb EJ, Metzler-Baddeley C, Aggleton JP (2018) The cingulum bundle: anatomy, function, and dysfunction. Neurosci Biobehav Rev 92:104–127. https://doi.org/10.1016/j.neubiorev.2018.05.008
Metoki A, Brookes RL, Zeestraten E et al (2017) Mnemonic function in small vessel disease and associations with white matter tract microstructure. Neuropsychologia 104:1–7. https://doi.org/10.1016/j.neuropsychologia.2017.07.027
van der Holst HM, Tuladhar AM, van Norden AG et al (2013) Microstructural integrity of the cingulum is related to verbal memory performance in elderly with cerebral small vessel disease: the RUN DMC study. Neuroimage 65:416–423. https://doi.org/10.1016/j.neuroimage.2012.09.060
Charlton RA, Barrick TR, Lawes IN et al (2010) White matter pathways associated with working memory in normal aging. Cortex 46(4):474–489. https://doi.org/10.1016/j.cortex.2009.07.005
Metzler-Baddeley C, Jones DK, Steventon J et al (2012) Cingulum microstructure predicts cognitive control in older age and mild cognitive impairment. J Neurosci 32(49):17612–17619. https://doi.org/10.1523/JNEUROSCI.3299-12.2012
Yu J, Lam CLM, Lee TMC (2017) White matter microstructural abnormalities in amnestic mild cognitive impairment: a meta-analysis of whole-brain and ROI-based studies. Neurosci Biobehav Rev 83:405–416. https://doi.org/10.1016/j.neubiorev.2017.10.026
Wang Z, Dai Z, Shu H et al (2017) Cortical thickness and microstructural white matter changes detect amnestic mild cognitive impairment. J Alzheimers Dis 56(1):415–428. https://doi.org/10.3233/JAD-160724
Remy F, Vayssiere N, Saint-Aubert L et al (2015) White matter disruption at the prodromal stage of Alzheimer’s disease: relationships with hippocampal atrophy and episodic memory performance. Neuroimage Clin 7:482–492. https://doi.org/10.1016/j.nicl.2015.01.014
Zhuang L, Sachdev PS, Trollor JN et al (2013) Microstructural white matter changes, not hippocampal atrophy, detect early amnestic mild cognitive impairment. PLoS ONE 8(3):e58887. https://doi.org/10.1371/journal.pone.0058887
Chen Y, Sha M, Zhao X et al (2017) Automated detection of pathologic white matter alterations in Alzheimer’s disease using combined diffusivity and kurtosis method. Psychiatry Res Neuroimaging 264:35–45. https://doi.org/10.1016/j.pscychresns.2017.04.004
Gong NJ, Wong CS, Chan CC et al (2013) Correlations between microstructural alterations and severity of cognitive deficiency in Alzheimer’s disease and mild cognitive impairment: a diffusional kurtosis imaging study. Magn Reson Imaging 31(5):688–694. https://doi.org/10.1016/j.mri.2012.10.027
Marrale M, Collura G, Brai M et al (2016) Physics, Techniques and review of neuroradiological applications of diffusion kurtosis imaging (DKI). Clin Neuroradiol 26(4):391–403. https://doi.org/10.1007/s00062-015-0469-9
Blair GW, Hernandez MV, Thrippleton MJ et al (2017) Advanced Neuroimaging of Cerebral Small Vessel Disease. Curr Treat Options Cardiovasc Med 19(7):56. https://doi.org/10.1007/s11936-017-0555-1
Winblad B, Palmer K, Kivipelto M et al (2004) Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256(3):240–246. https://doi.org/10.1111/j.1365-2796.2004.01380.x
Lu J, Li D, Li F et al (2011) Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol 24(4):184–190. https://doi.org/10.1177/0891988711422528
Baron J-C, Zhang X, Ding L et al (2016) Brain atrophy correlates with severe enlarged perivascular spaces in basal ganglia among lacunar stroke patients. PLoS ONE 11(2):e0149593. https://doi.org/10.1371/journal.pone.0149593
Ashburner J (2012) SPM: a history. Neuroimage 62(2):791–800. https://doi.org/10.1016/j.neuroimage.2011.10.025
Steven AJ, Zhuo J, Melhem ER (2014) Diffusion kurtosis imaging: an emerging technique for evaluating the microstructural environment of the brain. AJR Am J Roentgenol 202(1):W26-33. https://doi.org/10.2214/AJR.13.11365
Mori S, Oishi K, Jiang H et al (2008) Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40(2):570–582. https://doi.org/10.1016/j.neuroimage.2007.12.035
Ciesielska N, Sokołowski R, Mazur E et al (2016) Is the Montreal Cognitive Assessment (MoCA) test better suited than the mini-mental state examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatria Polska 50(5):1039–1052. https://doi.org/10.12740/PP/45368
Papma JM, de Groot M, de Koning I et al (2014) Cerebral small vessel disease affects white matter microstructure in mild cognitive impairment. Hum Brain Mapp 35(6):2836–2851. https://doi.org/10.1002/hbm.22370
Boyle PA, Yu L, Fleischman DA et al (2016) White matter hyperintensities, incident mild cognitive impairment, and cognitive decline in old age. Ann Clin Transl Neurol 3(10):791–800. https://doi.org/10.1002/acn3.343
Iadecola C, Duering M, Hachinski V et al (2019) Vascular cognitive impairment and dementia: JACC Scientific Expert Panel. J Am Coll Cardiol 73(25):3326–3344. https://doi.org/10.1016/j.jacc.2019.04.034
Uiterwijk R, van Oostenbrugge RJ, Huijts M et al (2016) Total cerebral small vessel disease MRI Score is associated with cognitive decline in executive function in patients with hypertension. Front Aging Neurosci 8:301. https://doi.org/10.3389/fnagi.2016.00301
Liu Q, Zhu Z, Teipel SJ et al (2017) White matter damage in the cholinergic system contributes to cognitive impairment in subcortical vascular cognitive impairment, no dementia. Front Aging Neurosci 9:47. https://doi.org/10.3389/fnagi.2017.00047
Peter J, Mayer I, Kammer T et al (2021) The relationship between cholinergic system brain structure and function in healthy adults and patients with mild cognitive impairment. Sci Rep 11(1):16080. https://doi.org/10.1038/s41598-021-95573-8
Bettcher BM, Mungas D, Patel N et al (2016) Neuroanatomical substrates of executive functions: beyond prefrontal structures. Neuropsychologia 85:100–109. https://doi.org/10.1016/j.neuropsychologia.2016.03.001
Kantarci K, Senjem ML, Avula R et al (2011) Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology 77(1):26–34. https://doi.org/10.1212/WNL.0b013e31822313dc
Sexton CE, Mackay CE, Lonie JA et al (2010) MRI correlates of episodic memory in Alzheimer’s disease, mild cognitive impairment, and healthy aging. Psychiatry Research: Neuroimaging 184(1):57–62. https://doi.org/10.1016/j.pscychresns.2010.07.005
Liu D, Li K, Ma X et al (2019) Correlations between the microstructural changes of the medial temporal cortex and mild cognitive impairment in patients with cerebral small vascular disease (cSVD): a diffusion kurtosis imaging study. Front Neurol 10:1378. https://doi.org/10.3389/fneur.2019.01378
Liu H, Liu D, Li K et al (2021) Microstructural changes in the cingulate gyrus of patients with mild cognitive impairment induced by cerebral small vessel disease. Neurol Res 43(8):659–667. https://doi.org/10.1080/01616412.2021.1910903
Kantarci K, Murray ME, Schwarz CG et al (2017) White-matter integrity on DTI and the pathologic staging of Alzheimer’s disease. Neurobiol Aging 56:172–179. https://doi.org/10.1016/j.neurobiolaging.2017.04.024
Nakata Y, Sato N, Nemoto K et al (2009) Diffusion abnormality in the posterior cingulum and hippocampal volume: correlation with disease progression in Alzheimer’s disease. Magn Reson Imaging 27(3):347–354. https://doi.org/10.1016/j.mri.2008.07.013
Wessa M, King AV, Meyer P et al (2016) Impaired and preserved aspects of feedback learning in aMCI: contributions of structural connectivity. Brain Struct Funct 221(5):2831–2846. https://doi.org/10.1007/s00429-015-1075-y
Damoiseaux JS, Smith SM, Witter MP et al (2009) White matter tract integrity in aging and Alzheimer’s disease. Hum Brain Mapp 30(4):1051–1059. https://doi.org/10.1002/hbm.20563
Li X, Wang H, Tian Y et al (2016) Impaired white matter connections of the limbic system networks associated with impaired emotional memory in Alzheimer’s disease. Front Aging Neurosci 8:250. https://doi.org/10.3389/fnagi.2016.00250
Cheng R, Chen L, Liu X et al (2020) Changes in gray matter asymmetries of the fusiform and parahippocampal gyruses in patients with subcortical ischemic vascular disease. Front Neurol 11:603977. https://doi.org/10.3389/fneur.2020.603977
Yin X, Han Y, Ge H et al (2013) Inferior frontal white matter asymmetry correlates with executive control of attention. Hum Brain Mapp 34(4):796–813. https://doi.org/10.1002/hbm.21477
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Our study was approved by the Ethics Committee of Civil Aviation General Hospital and Beijing Chaoyang Hospital of Capital Medical University, and informed consent was obtained from each patient before study. The patients/participants provided their written informed consent to participate in this study.
Conflict of interest
There were no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., Liu, D., Liu, M. et al. The microstructural abnormalities of cingulum was related to patients with mild cognitive impairment: a diffusion kurtosis imaging study. Neurol Sci 44, 171–180 (2023). https://doi.org/10.1007/s10072-022-06408-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06408-x