Abstract
There is growing concern that multiple sclerosis (MS) patients on certain therapies may be at higher risk for severe coronavirus disease 2019 (COVID-19). We conducted a systematic literature review to examine the available data on U.S. therapies approved to treat MS and the risk of SARS-CoV-2 infection or severe COVID-19 outcomes. We conducted searches in PubMed, Embase, and the WHO COVID-19 database through May 2, 2021, and retrieved articles describing clinical data on therapies approved to treat MS and the risk of infection with SARS-CoV-2 or the effects of such therapies on clinical outcomes of COVID-19. The literature search identified a total of 411 articles: 97 in PubMed, 227 in Embase, and 87 in the WHO database. After excluding duplicates and screening, we identified 15 articles of interest. We identified an additional article through a broader secondary weekly search in PubMed. Thus, ultimately, we reviewed 16 observational studies. Available data, which suggest that MS patients treated with anti-CD20 monoclonal antibodies may be at increased risk for severe COVID-19, are subject to relevant limitations. Generally, studies did not identify increased risk for COVID-19 worsening with other therapies approved to treat MS. Based on observational data, biological plausibility, novelty of the drug-event association, and public health implications in a subpopulation with potential impaired response to the COVID-19 vaccines, this safety signal merits further monitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is characterized by a wide range of clinical presentations, from asymptomatic to severe or fatal illness, sometimes with persistent debilitating symptoms. The strongest predictor for disease severity is age over 65 years [1]. Adults of any age with certain underlying medical conditions, such as cardiovascular diseases, diabetes, and chronic respiratory diseases, and those immunocompromised, are also at increased risk for severe illness [2]. Accordingly, special concerns exist regarding the severity of coronavirus disease 2019 (COVID-19) in patients with multiple sclerosis (MS) and the possible effects of certain therapies on COVID-19 outcomes [3]. The primary mechanism of action for therapies approved to treat MS is thought to be diminishing neuroinflammation by immunosuppression and immunomodulation effects. Thus, all therapies approved to treat MS modulate the immune system through mechanisms that include sequestration of lymphocytes, TH1/TH2 shift, interference with DNA synthesis in lymphocytes, depletion of immune cells, and/or changes in cytokine secretion pattern [4]. The effect of treatments on the immune system may be short-lasting or may last for months or even years [5]. Although some medications have a well-defined mechanism of action, for others, the mechanism remains poorly defined [4].
Multiple studies have evaluated the association between therapies approved to treat MS and COVID-19 risk and severity. Several of these studies reported an increased risk of severe COVID-19 outcomes in MS patients on anti-CD20 monoclonal antibody therapies [3, 6, 7]. In order to gain a more complete understanding of these emerging data, we conducted a systematic literature review to evaluate the available data on therapies approved to treat MS and the risk of SARS-CoV-2 infection or severe COVID-19 outcomes.
Methods
Search strategy
We focused on therapies approved to treat MS identified to have potential biological mechanisms relevant to SARS-CoV-2 infection and severity, namely, sphingosine-1-receptor (S1P) modulators (fingolimod, siponimod, ozanimod), monoclonal antibodies (natalizumab, alemtuzumab, and the anti-CD20 agents ocrelizumab and ofatumumab), fumarates (dimethyl fumarate, diroximel fumarate, monomethyl fumarate), teriflunomide, and cladribine. We did not include interferons or glatiramer acetate as we focused the search on therapies approved to treat MS specifically believed to increase the risk of infection, as reflected in their U.S. FDA labeling. We did not consider MS symptomatic therapies (e.g., dalfampridine) and therapies approved for MS exacerbations (e.g., acthar gel/corticotropin). Although we did not purposely aim to include rituximab, an anti-CD20 agent not approved for use as a treatment for MS in the United States and other many countries, some of the publications analyzed ocrelizumab and rituximab jointly or provided results for rituximab.
We conducted the capstone database search on January 28, 2021, in PubMed, Embase, and the WHO COVID-19 database and retrieved articles describing clinical data on therapies approved to treat MS and the risk of infection with SARS-CoV-2 or the effects of these therapies on clinical outcomes of COVID-19. We considered both published articles from peer-reviewed journals and articles posted online (at repositories such as MedRxiv.org) in advance of peer review. We repeated the same searches on April 4, 2021, and May 2, 2021, including data from January 1, 2021, through April 4, 2021, and through May 2, 2021, respectively (Table 1). We also conducted a secondary more sensitive weekly search in PubMed from January 10, 2021, through May 2, 2021 (Table 2).
Eligibility criteria
We included peer- and non-peer-reviewed articles meeting each of the two following criteria:
-
i.
Studies evaluating risk of SARS-CoV-2 infection and clinical course of COVID-19 with individual or class of drugs approved to treat MS
-
ii.
Studies that diagnosed COVID-19 based on symptoms or laboratory test results
Study selection
Two authors (SPV and MH) screened titles and abstracts to select articles that sought to provide a quantitative, inferential analysis of the association between therapies approved to treat MS and SARS-CoV-2 infection or COVID-19 severity. We excluded case reports, purely descriptive epidemiological studies, reviews, letters, and publications unrelated to the question of interest.
Data extraction and synthesis
Two or more epidemiologists (SPV, KL, AM, MH) performed full text review of each of the included studies. Then, the entire study team discussed each manuscript. After reaching consensus on the epidemiological assessment, the lead epidemiologist for each manuscript extracted descriptive and quantitative summary measures [first author, objective, study design, setting (data source, study period), study population, exposure, outcomes, results, additional comments] of the studies included in the systematic review (Supplemental Table 1), which were reviewed by all authors. We did not quantitatively summarize findings across studies.
Results
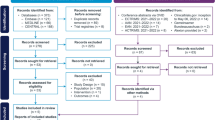
The literature search identified a total of 411 articles: 97 in PubMed, 227 in Embase, and 87 in the WHO COVID-19 database. After excluding duplicates (n = 199) and screening through review of the abstracts (or full manuscript when the articles did not include an abstract), we identified 15 articles of interest (Fig. 1). One additional article was identified through the secondary weekly search in PubMed. Thus, ultimately, we reviewed 16 observational studies [3, 6,7,8,9,10,11,12,13,14,15,16,17,18,19,20]. Summaries of the 16 observational studies included in the review are shown in Supplemental Table 1. As the mechanisms of action for therapies approved to treat MS and COVID-19 immunology are not well understood, it is challenging to classify them into distinct pharmacological groups [21]. For this review, we presented the results in three groups: (i) cell depletion (anti-CD20 monoclonal antibodies), (ii) immunomodulators (interferons and glatiramer acetate), and (iii) other therapies.
Anti-CD20 monoclonal antibodies
Several observational studies have identified a potential increased COVID-19 severity risk in MS patients being treated with anti-CD20 monoclonal antibodies. A study by Sormani et al. in an Italian cohort of 844 MS patients with SARS-CoV-2 infection found increased risk of severe COVID-19 in people treated with ocrelizumab or rituximab (OR, 2.37; 95% CI, 1.18–4.74) when compared to untreated individuals, after adjusting for region, age, sex, progressive MS course, Expanded Disability Status Scale (EDSS), disease duration, body mass index (BMI), comorbidities, and recent methylprednisolone use [7]. They found similar results when comparing anti-CD20 monoclonal antibodies to dimethyl fumarate (OR, 2.11; 95% CI, 1.03–4.33). The North American COViMS Registry by Salter et al. with 1,626 MS patients with SARS-CoV-2 infection identified an increased risk of hospitalization for COVID-19 in patients taking rituximab compared with those untreated (OR, 4.56; 95% CI, 2.10–9.90); although the point estimate for the risk of hospitalization for ocrelizumab was increased, the association was apparently not as strong as with rituximab (OR, 1.63; 95% CI, 0.98–2.72) [3]. A multi-country study by Simpson-Yap et al., published as a pre-print, which included 2,340 MS patients with SARS-CoV-2 infection, identified an increased risk for hospitalization, ICU admission, and ventilator use with rituximab (OR, 2.43; 95% CI, 1.48–4.02; OR, 3.93; 95% CI, 1.56–9.89; OR, 4.00; 95% CI, 1.54–10.39, respectively) and an increased risk for hospitalization with ocrelizumab (OR, 1.56; 95% CI:, 1.01–2.41), when compared to dimethyl fumarate; the risk for ICU admission with ocrelizumab was nearly significant (OR, 2.30; 95%, 0.98–5.39). The comparisons of rituximab and ocrelizumab with other pooled therapies, and also with natalizumab, showed similar trends for all outcomes investigated [6]. In a cross-sectional survey in Iran conducted by Safavi et al. among 712 MS patients, being on anti-CD20 therapies, compared to being on glatiramer acetate, interferons, dimethyl fumarate, or teriflunomide, was found associated with an increased risk for suspected COVID-19 (RR, 3.55; 95% CI, 1.45–8.68); suspected COVID-19 was identified by fever and cough or fever and shortness of breath or a presumptive diagnosis based on suggestive chest computed tomography [9]. Another relatively small study conducted in Poland by Czarnowska et al., which included 396 MS patients with confirmed SARS-CoV-2 infection being treated with therapies approved to treat MS, found that patients on ocrelizumab had a higher risk of COVID-19 hospitalization [18]. A U.S. study by Reder et al. using the IBM Explorys EHR database, which included 30,478 MS patients with an open prescription for a MS therapies—344 of them being SARS-CoV-2 infection positive—found that anti-CD20 therapy, when compared to fumarates, was associated with the highest risk for PCR-confirmed COVID-19 (OR, 3.25; 95% CI, 2.31–4.64) and also when compared to natalizumab (OR, 2.32; 95% CI, 1.56–3.57) and interferons (OR, 4.65; 95% CI, 3.23–6.82) [17]. In exploratory analyses, the Italian study also showed a trend for worse clinical outcomes with longer duration of anti-CD20 therapies when compared to other therapies [7]. The Spanish study by Zabalza et al., after adjusting for age, Barcelona residence, contact with a confirmed case, any comorbidity, and disease duration, found that time on anti-CD20 treatment (ocrelizumab or rituximab) was an independent risk factor for SARS-CoV-2 infection (OR per 2 years, 3.48; 95% CI, 1.44–8.45) [12] (Table 3, Supplemental Table 1).
A few studies did not find an association between anti-CD20 therapies use and SARS-CoV2 infection or severe COVID-19 (Table 3, Supplemental Table 1). A U.S. study by Kovvuru et al. with 42,899 patients with MS (115 COVID-19 cases) conducted in TriNetX, an electronic health record (EHR) database, did not identify differences in clinical outcomes of hospitalization, ICU care, intubation, or death between those on MS immunosuppressive therapies and those who were not [20]. The multi-center retrospective French study by Louapre et al. with 347 MS patients did not find an association of hospitalization for COVID-19 with anti-CD20 therapies, but its small sample size limited the ability to detect any associations [10]. Similarly, a relatively small study in Turkey among 309 MS patients with SARS-CoV-2 infection did not identify an increased risk of severe COVID-19 in people treated with rituximab or ocrelizumab when compared to fingolimod or natalizumab [19].
Interferons and glatiramer acetate
Because we focused on therapies specifically believed to increase the risk of infection, we did not include interferons or glatiramer acetate in the search strategy. Nevertheless, our search returned studies that analyzed risks with interferons and glatiramer acetate along with other therapies. In contrast to the studies of anti-CD20 monoclonal antibodies, some studies have identified a potential reduced risk of developing COVID-19 in MS patients being treated with interferons or glatiramer acetate (Table 4). However, definitive conclusions regarding a potential protective effect cannot be drawn without comparison to an appropriate untreated MS patient population. Reder et al. showed that MS patients with a prescription for interferon or glatiramer acetate in the Explorys database were less likely to develop COVID-19 than MS patients on any other MS therapy (0.61% vs. 1.27%; p < 0.0001 and 0.51 vs. 1.31%; p < 0.0001, respectively); when using fumarates as the reference, the adjusted odds ratios for COVID-19 with use of interferon-beta and glatiramer acetate were 0.70 (95% CI: 0.45–1.08) and 0.54 (0.34–0.84), respectively. The authors did not identify differences in risk with glatiramer acetate compared to interferons [17]. An updated analysis of the French cohort study presented at the MS Virtual Conference 2020 suggested a reduced risk of severe COVID-19 in patients treated with interferon-beta or glatiramer acetate [5].
Other therapies
A few studies have focused on other therapies approved to treat MS. A small cross-sectional study conducted in Italy, Spain, and Denmark by Dalla Costa et al. identified a trend for an increased risk of infection with alemtuzumab and cladribine when compared to interferons or glatiramer acetate, independent of age, sex, and disease course (OR, 3.78; 95% CI, 1.00–15.93). However, this study had limited power, did not account for relevant factors such as disability and comorbidities, used as comparators therapies for which protective effects on the risk of severe COVID-19 are presumed, and was subject to selection bias because those who were sicker and those who died (who might be on more aggressive immunosuppressive treatments) did not respond to the survey [16]. The larger studies by Salter et al., Simpson-Yap et al., and Sormani et al. did not identify increased risk for COVID-19 worsening with drugs other than anti-CD20 agents [3, 6, 7]. Sormani et al. also noted that recent use (less than one month prior to SARS-CoV-2 infection) of methylprednisolone was associated with a worse COVID-19 outcome (OR, 5.24; 95% CI, 2.20–12.53) [7]. Similarly, Salter et al. identified recent treatment (2 months prior to SARS-CoV-2 infection) with corticosteroids as a risk factor for increased COVID-19 severity. Studies in other populations have also shown that long-term corticosteroid use may increase the risk of COVID-19-related hospitalizations [22]. Thus, immunosuppression achieved with corticosteroids before infection may be a risk factor for a more severe infection, notwithstanding the therapeutic effects of corticosteroids in severe COVID-19 [23, 24].
Discussion
We found that current evidence suggests that MS patients treated with anti-CD20 monoclonal antibodies may be at increased risk for severe COVID-19. Generally, studies did not identify increased risk for COVID-19 worsening with other MS therapies. A few studies have identified a potential reduced risk of COVID-19 and severity in MS patients being treated with interferons or glatiramer acetate.
Four studies were notable for having more sophisticated methodology, even though three of these relied on voluntary reporting from treating neurologists and health care professionals. The Italian multi-center, registry-based, retrospective, observational study by Sormani et al., after adjusting for region, age, sex, MS disease phase (RRMS vs. progressive), EDSS, disease duration, BMI, presence of comorbidities, and recent methylprednisolone use, although not for covariates such as other prior immunomodulators and socioeconomic indicators, showed increased COVID-19 severity among MS patients with being treated with rituximab or ocrelizumab [7]. The authors used no therapy and fumarates as reference groups. The study also suggested increased COVID-19 severity among MS patients with advanced disease and disability and those on methylprednisolone in the month preceding the first symptoms of COVID-19 and, in exploratory analyses, an increasing trend with longer anti-CD20 therapy duration. The large multi-country, multi-center, retrospective, cross-sectional study by Simpson-Yap et al. identified consistent associations of rituximab with increased risk of hospitalization, ICU admission, and ventilator use and of ocrelizumab with hospitalization and ICU admission when using dimethyl fumarate, pooled therapies, and natalizumab as comparators [6, 25]. These analyses included adjustments for age, sex, MS phenotype, and disability, and additionally for comorbidities, BMI, and smoking in subgroup analyses where these data were available, although they did not adjust for covariates such as treatment duration, prior immunomodulation, and socioeconomic indicators. The North American registry-based, retrospective, cross-sectional study by Salter et al. identified increased disability as independently associated with worse clinical severity by using multivariable models that included a fixed set of covariates, including age, sex, race, ambulation, smoking history, glucocorticoids use, comorbidities, and therapies used to treat MS [3]. The authors also identified older age, Black race, cardiovascular comorbidities, obesity, and recent treatment with corticosteroids as risks factors for worse outcomes. Rituximab showed associations with worse outcomes compared to other therapies. Also, although the risk for hospitalization with ocrelizumab was also nominally increased, the association was not as strong as with rituximab. The fourth study was the EHR-based, retrospective cohort study by Reder et al., in which the authors by using fumarates as the comparator and adjustments for age, sex, BMI, comorbidities, and race found that patients with MS were less likely to develop COVID-19 when treated with interferons and glatiramer acetate but more likely when treated with rituximab or ocrelizumab [17].
Similarly to what has been seen in the general population, MS patients at increased risk for severe COVID-19 tend to be older and have more comorbidities and higher disability [3, 6, 7, 10, 25]. Progressive MS phenotype also appears to be associated with higher COVID-19 severity [10, 25]. On the other hand, younger patients, those required to work on site, and those with a lower level of disability might be at higher exposure risk [12, 14]. In observational studies, when comparing MS patients with untreated individuals or with individuals treated with other therapies, disentangling the effects of modification of immunity from differences in patient and disease characteristics, including, but not limited to, age, relevant comorbidities, MS disease course, treatment duration, prior immunomodulation, disability, and higher risk activities, as well as their potential interactions, is particularly challenging.
Biological plausibility
An effective immune response against SARS-CoV-2 infection requires both arms of the immune system, innate and adaptive [26, 27].
Anti-CD20 monoclonal antibody therapies are not expected to affect responses of the innate immune system, which are critical for initial viral control [28]. They selectively bind to B-cells that express CD20 antigen, leading to B-cell depletion mediated through complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity [4, 21]. Additionally, these therapies also target a subpopulation of T-cells expressing CD20 on their surface [29]. Importantly, anti-CD20 monoclonal antibodies reduce type II pneumocyte response to infections and prevent CD4 + T-cell priming, thereby attenuating the clearance of viral infections from the respiratory tract [21]. Anti-CD20 therapies also impact directly on the production of IL-6 by B cells [5]; it has even been suggested that a moderately reduced immune response due to lack of peripheral B cells might play a favorable role on patients on these therapies [28]. In this review, rituximab, which is often used as an off-label medication to treat MS, has been found associated with an apparent higher increased risk for COVID-19 worsening than ocrelizumab [3, 6]. Salter et al. argued that, in their study, the differences might be due to longer treatment duration with rituximab because ocrelizumab was more recently available [3]. At least in the United States, these differences could also be influenced by differential socioeconomic status and access to care (FDA approved a rituximab biosimilar in 2018).
Immunomodulatory effects of interferon beta-1b include the enhancement of suppressor T cell activity, reduction of pro-inflammatory cytokine production, downregulation of antigen presentation, and inhibition of lymphocyte trafficking into the central nervous system [30]. Based on the antiviral effects of type I interferon, and on the dampening of type I interferon responses in the host by the SARS-CoV-2 infection, MS patients who are being treated with interferon-beta are hypothesized to be at lower risk for COVID-19 or its severe forms [5, 21]. This hypothesis is aligned with the results from a recently published study by Sormani et al. using updated data from Italy and France [7, 10], in which interferon was associated with decreased COVID-19 severity [31]. The same study also showed increased COVID-19 severity with anti-CD20 therapies. The mechanisms by which glatiramer acetate exerts its effects in patients with MS are not fully understood. Treatment with glatiramer acetate may interfere with the recognition of foreign antigens in a way that would undermine defenses against infection. There is no evidence that glatiramer acetate does this, but there has not been a systematic evaluation of this risk [32]. It has been suggested that glatiramer acetate causes a shift from a pro-inflammatory to an anti-inflammatory response. Also, glatiramer acetate blocks IFN-γ-mediated activation of macrophages, which is thought to play an essential role in acute respiratory distress syndrome (ARDS) [28].
Fumarates also have a less well-understood therapeutic mechanism of action [4]. While fumarates may decrease lymphocyte counts, treated patients have functional T and B cells; have stable serum IgA, IgG, IgM, and IgG1-4 over 2 years of treatment; and have adequate seroprotective responses to inactivated vaccines [17, 33]. In vitro studies showed that dimethyl fumarate blocks pro-inflammatory cytokine production and can inhibit macrophage function. Thus, this immunomodulatory effect could be potentially beneficial [28].
S1P modulators bind 1 of the 5 subtypes of S1P receptors resulting in internalization of the receptor and sequestration of lymphocytes in lymph nodes. S1P modulators are also able to cross the blood–brain barrier, where they may have other direct effects [4]. Fingolimod is associated with an increased risk of mild infections, mainly involving the lower respiratory tract and increased risk for herpes virus infections or reactivations [28]. A possible beneficial effect on COVID-19 is predicted by studies showing that S1P signaling is involved in mediating lung injury in ARDS and that fingolimod promotes the integrity of the pulmonary endothelial barrier [5, 28].
Cladribine is a purine analogue taken up into rapidly proliferating cells such as lymphocytes and incorporated into DNA, leading to cell death [4]. The effect of cladribine is mainly on CD4 + and CD8 + T cells, and also B cells. Accordingly, transient lymphopenia (most often mild to moderate) is a common adverse event. The effects on innate immune cells such as neutrophils, monocytes, and NK cells are minor. Due to the lymphopenia, the risk of infection with SARS-CoV-2 may be increased in patients on cladribine [28].
Teriflunomide, an active metabolite of leflunomide, selectively and reversibly inhibits the mitochondrial enzyme dihydroorotate dehydrogenase, impairing pyrimidine synthesis, resulting in a cytostatic effect on proliferating lymphocytes [4]. It reduces immune activation without significant immunosuppression. This function may be potentially beneficial in SARS-CoV-2 infection and may prevent an excessive host immune response. Moreover, teriflunomide may decrease SARS-CoV-2 replication inside the infected cell. Thus, it may be useful through both antiviral and immunomodulatory effects [5, 28].
Natalizumab is a humanized monoclonal antibody that selectively binds α4 integrin subunit expressed on the surface of lymphocytes, preventing the migration of lymphocytes into the CNS [4]. Its treatment is associated with increased risk of progressive multifocal leukoencephalopathy (PML) in seropositive patients to the John Cunningham (JC) virus, although extended interval dosing regimens have greatly decreased such risk [5]. Based on the recent studies showing that SARS-CoV-2 may use integrin to enter the human cells [34], natalizumab as an antibody against α4 integrin might be protective against the infection [28].
Alemtuzumab is a fully humanized monoclonal antibody that selectively binds CD52 antigen on lymphocytes, leading to T and B-lymphocyte depletion [4]. It acts via antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. It also activates pro-apoptotic pathways on CD52-expressing cells. Given the lymphopenia, the incidence of infection in the early months after treatment with alemtuzumab is high. Following the initial depletion, alemtuzumab produces a lymphocyte reconstitution from a new lineage [28].
Limitations
The observational studies included in this review present limitations that should be taken into account when interpreting the results. The large majority of studies had limited sample size, which limited the ability to detect associations; assess the effect of the different MS therapy classes on more severe outcomes, such as requirement for ventilation, ICU stays, or death; analyze the MS therapies independently; and/or conduct relevant adjustments [7,8,9,10,11,12, 14, 16,17,18,19,20]. Studies conducted in EHR databases may have missed out-of-network COVID-19 diagnoses and outcomes; also, data completeness may be affected by the high medical workload during the pandemic [17, 20]. Most studies were subject to selection bias—some of them analyzed younger and healthier populations and missed patients with higher degree of neurological disability and hospitalized patients [8, 9, 12,13,14, 16, 18, 19]; others used reporting of COVID-19 cases who had been in contact with their neurologists and, therefore, may have selected more moderate-to-severe cases (i.e., underreporting of mild cases), individuals receiving therapies with the stronger immunosuppressive effects, and/or those with more frequent contacts with treating neurologists [3, 6, 7, 10, 25]; fatal cases were not represented or were likely underrepresented in these studies [3, 6,7,8,9,10, 12,13,14, 16, 18, 19]. Also, if the factors that influence sample selection also influence the variables of interest, the relationship between these variables of interest can become distorted [35]. In these latter studies, collider bias may relate to greater proportions of people who have experienced an event such as hospitalization with COVID-19, have volunteered their participation in a large-scale study, or have been tested for active infection—who particularly early in the pandemic, were likely patients showing severe symptoms, patients at higher risk of infection and severe illness, or patients who were tested due to MS and/or its immunosuppressive treatment. Also, in some settings, physician’s decisions to hospitalize MS patients with SARS-CoV-2 infection might have been influenced not only by the clinical condition, but also by the MS coexistence [18] and the use of therapies with the stronger immunosuppressive effects. This could represent a threat to the internal validity of the study findings because associations induced by collider bias are properties of the sample rather than the individuals that comprise it [35]. None of the reviewed studies included information on therapeutic management that might have influenced COVID-19 clinical progression. Lastly, since some studies analyzed therapies approved to treat MS with presumed beneficial effects on COVID-19 risk and severity (e.g., interferon-beta) [9, 16] and therapies with markedly different mechanisms of action, specifically in regard to immunosuppressive effects on COVID-19 risk [8,9,10, 12, 14, 19, 20], results aggregated across treatments could reflect heterogeneous results.
Conclusions
Despite their limitations, available data at the time of this review suggested increased COVID-19 severity among MS patients with advanced disease and disability [7, 10], those being treated with anti-CD20 therapies (rituximab or ocrelizumab) [3, 6, 7, 17, 25], and those with recent use of glucocorticoids [7]. It also suggested that patients with MS were less likely to acquire PCR-confirmed COVID-19 when treated with interferons and glatiramer acetate [17].
With the advent of effective immunizations against COVID-19, there are emerging data on responsiveness to vaccinations in patients treated with immunosuppressive therapies, including therapies approved to treat MS. Despite recently published studies showing impaired humoral response to mRNA COVID-19 vaccines in patients treated with certain therapies approved to treat MS, it remains possible that an adequate T cell vaccine response could still occur that leads to protection [36,37,38,39,40,41]. Ongoing studies of immune response to COVID-19 vaccines and COVID-19 vaccine effectiveness in patients on therapies approved to treat MS may help clarify the question.
In conclusion, available data, which suggest that MS patients treated with anti-CD20 monoclonal antibodies may be at increased risk for severe COVID-19, are subject to relevant limitations. It appears that other therapies approved to treat MS are not associated with increased COVID-19 risk and severity. A few studies have indicated a potential reduced risk of COVID-19 and clinical severity in MS patients being treated with interferons or glatiramer acetate. Based on observational data, biological plausibility, novelty of the drug-event association, and public health implications in a subpopulation with potential impaired response to the COVID-19 vaccines, this safety signal merits further monitoring.
Data availability
Not applicable.
Code availability
Not applicable.
Change history
15 January 2022
The Supplementary materials has been updated
References
Brodin P (2021) Immune determinants of COVID-19 disease presentation and severity. Nat Med 27(1):28–33
Centers for Disease Control and Prevention (2021) COVID-19: People with certain medical conditions. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed 26 May 2021
Salter A et al (2021) Outcomes and risk factors associated with SARS-CoV-2 infection in a North American Registry of patients with multiple sclerosis. JAMA Neurol 78(6):699–708
McGinley MP, Goldschmidt CH, Rae-Grant AD (2021) Diagnosis and treatment of multiple sclerosis: a review. JAMA 325(8):765–779
Laroni A et al (2020) COVID-19 in patients with multiple sclerosis undergoing disease-modifying treatments. Mult Scler 27(14):2126–2136. https://doi.org/10.1177/1352458520971817
Simpson-Yap S et al (2021) Associations of DMT therapies with COVID-19 severity in multiple sclerosis. medRxiv :2021.02.08.21251316. https://doi.org/10.1101/2021.02.08.21251316
Sormani MP et al (2021) Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol 89(4):780–789
Mantero V et al (2020) Assessing the susceptibility to acute respiratory illness COVID-19-related in a cohort of multiple sclerosis patients. Mult Scler Relat Disord 46:102453
Safavi F, Nourbakhsh B, Azimi AR (2020) B-cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. Mult Scler Relat Disord 43:102195
Louapre C et al (2020) Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 77(9):1079–1088
Chaudhry F et al (2020) COVID-19 in multiple sclerosis patients and risk factors for severe infection. J Neurol Sci 418:117147
Zabalza A et al (2020) COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. Eur J Neurol 28(10):3384–3395. https://doi.org/10.1111/ene.14690
Sahraian MA et al (2020) Evaluation of the rate of COVID-19 infection, hospitalization and death among Iranian patients with multiple sclerosis. Mult Scler Relat Disord 46:102472
Moss BP et al (2020) Multiple sclerosis management during the COVID-19 pandemic. Mult Scler J 26(10):1163–1171
Capasso N et al. (2020) Prevalence of SARS-CoV-2 antibodies in multiple sclerosis: the hidden part of the Iceberg. J Clin Med 9(12):4066. https://doi.org/10.3390/jcm9124066
Dalla Costa G et al (2020) Real-time assessment of COVID-19 prevalence among multiple sclerosis patients: a multicenter European study. Neurol Sci 41(7):1647–1650
Reder AT et al (2021) COVID-19 in patients with multiple sclerosis: associations with disease-modifying therapies. CNS Drugs 35(3):317–330
Czarnowska A et al (2021) Clinical course and outcome of SARS-CoV-2 infection in multiple sclerosis patients treated with disease-modifying therapies - the Polish experience. Neurol Neurochir Pol 55(2):212–222
Sen S et al (2021) The outcome of a national MS-Covid-19 study: what the Turkish MS cohort reveals? Mult Scler Relat Disord 52:102968
Kovvuru S et al (2021) Immunosuppression in chronic autoimmune neurological disorders during the COVID-19 pandemic. J Neurol Sci 420:117230
Chaudhry F et al (2021) Review of the COVID-19 risk in multiple sclerosis. J Cell Immunol 3(2):68–77
Gianfrancesco M et al (2020) Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 79(7):859–866
W. H. O. Rapid Evidence Appraisal for COVID-19 Therapies Working Group, Sterne JAC, Murthy S et al (2020) Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 324:1330–41
Group, R.C., et al (2021) Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 384(8):693–704
Simpson-Yap S et al (2021) Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology 97(19):e1870–e1885
Schultze JL, Aschenbrenner AC (2021) COVID-19 and the human innate immune system. Cell 184(7):1671–1692
Sette A, Crotty S (2021) Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 184(4):861–880
Sharifian-Dorche M et al (2021) COVID-19 and disease-modifying therapies in patients with demyelinating diseases of the central nervous system: a systematic review. Mult Scler Relat Disord 50:102800
Frisch ES, Pretzsch R, WeberMS, (2021) A Milestone in Multiple Sclerosis Therapy: Monoclonal Antibodies Against CD20-Yet Progress Continues. Neurotherapeutics 18(3):1602–1622. https://doi.org/10.1007/s13311-021-01048-z
US Food and Drug Administration (2021) Betaseron (beta interferon 1b). US labeling dated October 1, 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/103471s5197lbl.pdf. Accessed 9 May 2021
Sormani MP, Salvetti M, Labauge P et al (2021) DMTs and Covid-19 severity in MS: a pooled analysis from Italy and France. Ann Clin Transl Neurol 8(8):1738–1744. https://doi.org/10.1002/acn3.51408
US Food and Drug Administration 2020 Copaxone (glatiramer acetate injection). US labeling dated July 22, 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020622s110lbl.pdf. Accessed 9 May 2021
von Hehn C et al (2018) Immune response to vaccines is maintained in patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm 5(1):e409
Makowski L, Olson-Sidford W, W-Weisel J (2021) Biological and Clinical Consequences of Integrin Binding via a Rogue RGD Motif in the SARS CoV-2 Spike Protein. Viruses 13(2):146. https://doi.org/10.3390/v13020146
Griffith GJ et al (2020) Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun 11(1):5749
Achiron A et al (2021) Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord 14:17562864211012836
Ali A et al (2021) Characterization of humoral response to COVID mRNA vaccines in multiple sclerosis patients on disease modifying therapies. Vaccine 39(41):6111–6116. https://doi.org/10.1016/j.vaccine.2021.08.078
Brill L et al. (2021) Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol 78(12):1510-1514. https://doi.org/10.1001/jamaneurol.2021.3599
Madelon N et al (2021) Robust T cell responses in anti-CD20 treated patients following COVID-19 vaccination: a prospective cohort study. Clin Infect Dis. https://doi.org/10.1093/cid/ciab954
Disanto G et al (2021) Association of disease-modifying treatment and anti-CD20 infusion timing with humoral response to 2 SARS-CoV-2 vaccines in patients with multiple sclerosis. JAMA Neurol 78(12):1529-1531. https://doi.org/10.1001/jamaneurol.2021.3609
Tortorella C et al. (2021) Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology. https://doi.org/10.1212/WNL.0000000000013108
Funding
This project was supported in part by an appointment to the ORISE Research Participation Program at the Center for Drug Evaluation and Research (CDER) administered by the Oak Ridge Institute for Science and Education through an agreement between the U.S. Department of Energy and CDER.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Informed consent
Not applicable.
Disclaimer
This article reflects the views of the authors and should not be construed to represent the U.S. Food and Drug Administration’s views or policies.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manila Hada contributed to this research as a participant in the ORISE Research Fellowship Program at the Center for Drug Evaluation and Research, U.S. Food and Drug Administration
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hada, M., Mosholder, A.D., Leishear, K. et al. Systematic review of risk of SARS-CoV-2 infection and severity of COVID-19 with therapies approved to treat multiple sclerosis. Neurol Sci 43, 1557–1567 (2022). https://doi.org/10.1007/s10072-021-05846-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05846-3