Abstract
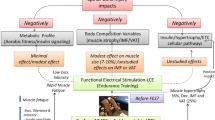
Muscle atrophy is a great consequence of spinal cord injuries (SCI) due to immobility. SCI’s detrimental effects on large muscle groups may lead to secondary effects such as glucose intolerance, increased risk of metabolic syndrome, and diabetes. Exercising with blood flow restriction (BFR) has been proposed as an effective method to induce hypertrophy using low training loads, with little or no muscle damage. This study investigated acute and chronic effects of low-intensity functional electrical stimulation (FES) combined with BFR on muscles affected by spinal cord injury. The acute effects of one bout of FES with (FES + BFR group) and without BFR (FES group) on muscle thickness (MT) and edema formation were compared. The chronic effects on MT and edema following 8 weeks of twice weekly training with and without BFR were also compared. The FES + BFR group showed MT and edema increases compared to the FES only group (p< 0.05). The FES + BFR showed a chronic MT increase after 4 weeks of training (p <0.05), with no further MT increases from the 4th to the 8th week (p>0.05). Following 3 weeks of detraining, MT decreased to baseline. No MT changes were observed in the FES (p>0.05). The FES + BF stimuli induced MT increases on the paralyzed skeletal muscles of SCI. The acute effects suggest that FES causes a greater metabolite accumulation and edema when combined with BFR. The early increases in MT can be attributed to edema, whereas after the 4th week, it is likely to be related to muscle hypertrophy. Register Clinical Trial Number on ReBeC: RBR-386rm8




Similar content being viewed by others
Data availability
Upon request
Code availability
not applicable
References
Lundell LS, Savikj M, Kostovski E, Iversen PO, Zierath JR, Krook A et al (2018) Protein translation, proteolysis and autophagy in human skeletal muscle atrophy after spinal cord injury. Acta Physiol (Oxf) England 223:e13051
Castro MJ, Apple DF, Hillegass EA, Dudley GA (1999) Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol 80:373–378
Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA (2004) Intramuscular fat and glucose tolerance after spinal cord injury - a cross-sectional study. Spinal Cord 42:711–716
Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Gater DR (2015) The effects of electrical stimulation on body composition and metabolic profile after spinal cord injury - part I. J Spinal Cord Med 38:23–37
Gorgey AS, Shepherd C (2010) Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: Case report. J Spinal Cord Med 33:90–95
Kasper K (2019) Sports Training Principles. Curr Sports Med Rep 18:95–96
Gerrits HL, De Haan A, Hopman MTE, van Der Woude LHV, Jones DA, Sargeant AJ (1999) Contractile properties of the quadriceps muscle in individuals with spinal cord injury. Muscle Nerve 22:1249–1256
Tidball JG (2011) Mechanisms of muscle injury, repair, and regeneration. Compr Physiol 1:2029–2062
Iversen PO, Hjeltnes N, Holm B, Flatebo T, Strom-Gundersen I, Ronning W et al (2000) Depressed immunity and impaired proliferation of hematopoietic progenitor cells in patients with complete spinal cord injury. Blood. 96:2081–2083
Patterson SD, Leggate M, Nimmo MA, Ferguson RA (2013) Circulating hormone and cytokine response to low-load resistance training with blood flow restriction in older men. Eur J Appl Physiol 113:713–719
Bickel CS, Slade JM, Dudley GA (2004) Long-term spinal cord injury increases susceptibility to isometric contraction-induced muscle injury. Eur J Appl Physiol 91:308–313
Loenneke JP, Wilson JM, Marín PJ, Zourdos MC, Bemben MG (2012) Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol 112:1849–1859
Takarada Y, Nakamura Y, Aruga S, Onda T, Miyazaki S, Ishii N (2000) Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol 88:61–65
Laurentino GC, Ugrinowitsch C, Roschel H, Aoki MS, Soares AG, Neves M et al (2012) Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc. [cited 2021 Feb 18];44:406–12. Available from: http://journals.lww.com/00005768-201203000-00006
Loenneke JP, Fahs CA, Rossow LM, Abe T, Bemben MG (2012) The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med Hypotheses. Elsevier Ltd; 78:151–4. Available from: https://doi.org/10.1016/j.mehy.2011.10.014
Ozaki H, Sakamaki M, Yasuda T, Fujita S, Ogasawara R, Sugaya M et al (2011) Increases in thigh muscle volume and strength by walk training with leg blood flow reduction in older participants. J Gerontol A Biol Sci Med Sci. United States 66:257–263
Thiebaud R, Loenneke JP, Fahs CA, Kim D, Ye X, Abe T, Nosaka K, Bemben MG (2014) Muscle damage after low-intensity eccentric contractions with blood flow restriction. Acta Physiol Hung 101:150–157
Farup J, de Paoli F, Bjerg K, Riis S, Ringgard S, Vissing K (2015) Blood flow restricted and traditional resistance training performed to fatigue produce equal muscle hypertrophy. Scand J Med Sci Sports 25:754–763
Natsume T, Ozaki H, Saito AI, Abe T, Naito H (2015) Effects of electrostimulation with blood flow restriction on muscle size and strength. Med Sci Sports Exerc. United States 47:2621–2627
Gorgey AS, Timmons MK, Dolbow DR, Bengel J, Fugate-Laus KC, Michener LA et al (2016) Electrical stimulation and blood flow restriction increase wrist extensor cross-sectional area and flow meditated dilatation following spinal cord injury. Eur J Appl Physiol. Springer Berlin Heidelberg 116:1231–1244
Bosy-Westphal A, Reinecke U, Schlörke T, Illner K, Kutzner D, Heller M, Müller MJ (2004) Effect of organ and tissue masses on resting energy expenditure in underweight, normal weight and obese adults. Int J Obes 28:72–79
Andrade SF, Skiba GH, Krueger E, Rodacki AF (2016) Effects of Electrostimulation with blood flow restriction on muscle thickness and strength of the soleus. J Exerc Physiol Online 19:59–69
Nosaka K, Aldayel A, Jubeau M, Chen TC (2011) Muscle damage induced by electrical stimulation. Eur J Appl Physiol 2427–37
Natsume T, Ozaki H, Saito AI, Abe TNH (2015) Effects of electrostimulation with blood flow restriction on muscle size and strength. Med Sci Sport Exerc 2621–7
Hopkins WG (2000) The author’s reply. Sports Med 30:375–378
Weir JP (2005) Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. United States 19:231–240
Lixandrão ME, Ugrinowitsch C, Bottaro M, Chacon-Mikahil MPT, Cavaglieri CR, Min LL et al (2014) Vastus lateralis muscle cross-sectional area ultrasonography validity for image fitting in humans. J Strength Cond Res. United States 28:3293–3297
Damas F, Phillips SM, Lixandrão ME, Vechin FC, Libardi CA, Roschel H et al (2016) Early resistance training-induced increases in muscle cross-sectional area are concomitant with edema-induced muscle swelling. Eur J Appl Physiol. Springer Berlin Heidelberg 116:49–56
Rabischong E, Ohanna F (1992) Effects of functional electrical stimulation (FES) on evoked muscular output in paraplegic quadriceps muscle. Paraplegia. England 30:467–473
Elbasiouny SM, Moroz D, Bakr MM, Mushahwar VK (2010) Management of spasticity after spinal cord injury: current techniques and future directions. Neurorehabil Neural Repair 24:23–33
Fritz CO, Morris PE, Richler JJ (2012) Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen 141:2–18
Cohen J (2013) Statistical power analysis for the behavioral sciences. Acad Press
Joyner MJ, Casey DP (2015) Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95:549–601
Kristensen JK, Henriksen O (1980) Excess cumulative blood flow and repayment during reactive hyperemia in human cutaneous tissue. Acta Physiol Scand 108:1–6
Smith LW, Smith JD, Criswell DS (2002) Involvement of nitric oxide synthase in skeletal muscle adaptation to chronic overload. J Appl Physiol 92:2005–2011
Yanagisawa O, Niitsu M, Yoshioka H, Goto K, Kudo H, Itai Y (2003) The use of magnetic resonance imaging to evaluate the effects of cooling on skeletal muscle after strenuous exercise. Eur J Appl Physiol 89:53–62
Koller A, Bagi Z (2002) On the role of mechanosensitive mechanisms eliciting reactive hyperemia. Am J Physiol Heart Circ Physiol 283:2250–2259
Watson PD, Garner RP, Ward DS (1993) Water uptake in stimulated cat skeletal muscle. Am J Phys Regul Integr Comp Phys 264:790–796
Fleckenstein JL, Haller RG, Lewis SF, Archer BT, Barker BR, Payne J, Parkey RW, Peshock RM (1991) Absence of exercise-induced MRI enhancement of skeletal muscle in McArdle’s disease. J Appl Physiol 71:961–969
Loenneke JP, Kim D, Fahs CA, Thiebaud RS, Abe T, Larson RD et al (2016) The influence of exercise load with and without different levels of blood flow restriction on acute changes in muscle thickness and lactate. Clin Physiol Funct Imaging 37:734–740
Olive JL, Dudley GA, McCully KK (2003) Vascular remodeling after spinal cord injury. Med Sci Sports Exerc 35:901–907
Venturelli M, Amann M, Layec G, Mcdaniel J, Trinity JD, Fjeldstad AS et al (2014) Passive leg movement-induced hyperaemia with a spinal cord lesion: evidence of preserved vascular function. Acta Physiol 210:429–439
Frigeri A, Nicchia GP, Verbavatz JM, Valenti G, Svelto M (1998) Expression of aquaporin-4 in fast-twitch fibers of mammalian skeletal muscle. J Clin Invest 102:695–703
Hornberger TA, Esser KA (2004) Mechanotransduction and the regulation of protein synthesis in skeletal muscle. Proc Nutr Soc 63:331–335
Miranda AR, Hassouna HI (2000) Mechanisms of thrombosis in spinal cord injury. Hematol Oncol Clin North Am 14:401–416
Loenneke JP, Thiebaud RS, Fahs CA, Rossow LM, Abe T, Bemben MG (2013) Blood flow restriction pressure recommendations: a tale of two cuffs. Front Physiol 33:325–327
Vechin FECV, Ibardi CLAL, Onceic MISC, Ixandra MAEL, Avaglieri CLRC, Atricia MARAP et al (2015) and H Igh -I Ntensity R Esistance T Raining on. J Strength Cond Res 29:1071–1076
Loenneke JP, Wilson JM, Wilson GJ, Pujol TJ, Bemben MG (2011) Potential safety issues with blood flow restriction training. Scand J Med Sci Sports. Denmark 21:510–518
Triolo RJ, Moss JD, Bhadra N (2001) A reusable, self-adhesive electrode for intraoperative stimulation in the lower limbs. J Rehabil Res Dev 38:527–532
Levy M, Mizrahi J, Susak Z (1990) Recruitment, force and fatigue characteristics of quadriceps muscles of paraplegics isometrically activated by surface functional electrical stimulation. J Biomed Eng 12:150–156
Abe T, Beekley MD, Hinata S, Koizumi K, Sato Y (2005) Day-to-day change in muscle strength and MRI-measured skeletal muscle size during 7 days KAATSU resistance training: A case study. Int J KAATSU Train Res 1:71–76
Abe T, Kawamoto K, Yasuda T, Kearns C, Midorikawa T, Sato Y (2005) Eight days KAATSU-resistance training improved sprint but not jump performance in collegiate male track and field athletes. Int J Kaatsu Train Res 1:19–23
Yasuda T, Abe T, Sato Y, Midorikawa T, Kearns CF, Inoue K, Ryushi T, Ishii N (2005) Muscle fiber cross-sectional area is increased after two weeks of twice daily KAATSU-resistance training. Int J KAATSU Train Res 1:65–70
Fujita T, Brechue WF, Kurita K, Sato Y, Abe T (2008) Increased muscle volume and strength following six days of low-intensity resistance training with restricted muscle blood flow. Int J KAATSU Train Res 4:1–8
Damas F, Phillips SM, Lixandrão ME, Vechin FC, Libardi CA, Roschel H et al (2016) An inability to distinguish edematous swelling from true hypertrophy still prevents a completely accurate interpretation of the time course of muscle hypertrophy. Eur J Appl Physiol. Springer Berlin Heidelberg 116:445–446
Wernbom M, Paulsen G, Nilsen TS, Hisdal J, Raastad T (2012) Contractile function and sarcolemmal permeability after acute low-load resistance exercise with blood flow restriction. Eur J Appl Physiol 112:2051–2063
Thiebaud RS, Yasuda T, Loenneke JP, Abe T (2013) Eff ects of low-intensity concentric and eccentric exercise combined with blood fl ow restriction on indices of exercise-induced muscle damage. 5:53–9
Murphy E, Steenbergen C (2008) Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. 581–609.
Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG (2012) IL-10 Triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol 189:3669–3680
Häkkinen K, Alen M, Kallinen M, Newton RU, Kraemer WJ (2000) Neuromuscular adaptation during prolonged strength training, detraining and re-strength-training in middle-aged and elderly people. Eur J Appl Physiol. Germany 83:51–62
Elliott B, Renshaw D, Getting S, Mackenzie R (2012) The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol 205:324–340
Verdijk LB, Dirks ML, Snijders T, Prompers JJ, Beelen M, Jonkers RAM et al (2012) Reduced satellite cell numbers with spinal cord injury and aging in humans. Med Sci Sports Exerc 44:2322–2330
Carlson CJ, Booth FW, Gordon SE (1999) Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading. Am J Phys Regul Integr Comp Phys 277:601–606
Burgomaster KA, Moore DR, Schofield LM, Phillips SM, Sale DG, Gibala MJ (2003) Resistance training with vascular occlusion: metabolic adaptations in human muscle. Med Sci Sports Exerc 35:1203–1208
Chan ST, Johnson AW, Moore MH, Kapadia CR, Dudley HA (1982) Early weight gain and glycogen-obligated water during nutritional rehabilitation. Hum Nutr Clin Nutr. England 36:223–232
Ribeiro AS, Avelar A, Schoenfeld BJ, Ritti Dias RM, Altimari LR, Cyrino ES (2014) Resistance training promotes increase in intracellular hydration in men and women. Eur J Sport Sci. England 14:578–585
Fluckey JD, Ploug T, Galbo H (1999) Mechanisms associated with hypoxia- and contraction-mediated glucose transport in muscle are fibre-dependent. Acta Physiol Scand 167:83–87
Tesch PA, Karlsson J (1985) Muscle fiber types and size in trained and untrained muscles of elite athletes. J Appl Physiol 59:1716–1720
Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ (2009) Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Phys Cell Physiol 296:1258–1270
Drummond MJ, Glynn EL, Lujan HL, Dicarlo SE, Rasmussen BB (2008) Gene and protein expression associated with protein synthesis and breakdown in paraplegic skeletal muscle. Muscle Nerve 37:505–513
Funding
Self-funded.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design and implementation of the research and to the analysis of the results. Skiba wrote the paper, manufactured the figures, and acquired samples for research. Rodacki supervised the project.
Corresponding author
Ethics declarations
Ethics approval
CAAE NUMBER: 48571315.4.0000.5225 OPINION NUMBER: 1.474.654
Informed consent statement
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Consent to participate
All participants signed an informed consent form that was approved by the ethics committee.
Consent for publication
All authors are aware and agree with the contents of the manuscript.
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Skiba, G.H., Andrade, S.F. & Rodacki, A.F. Effects of functional electro-stimulation combined with blood flow restriction in affected muscles by spinal cord injury. Neurol Sci 43, 603–613 (2022). https://doi.org/10.1007/s10072-021-05307-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05307-x